Biotechnology
BIOTECHNOLOGY
Biotechnology is the application of biological science and engineering techniques to develop new products derived from biological materials, such as vaccines, enzymes, antibiotics, and food. It plays a crucial role in various industries, including healthcare, agriculture, environmental conservation, and industrial manufacturing.
The advancement of modern biotechnology has been made possible by two fundamental fields:
1. Genetic Engineering – This field involves techniques that allow scientists to modify the genetic material (DNA and RNA) of an organism. By altering the genetic composition of living cells, genetic engineering enables the introduction of desirable traits, such as disease resistance in crops or the production of therapeutic proteins in bacteria. Through recombinant DNA technology, scientists can insert genes into host organisms, allowing them to express specific characteristics that benefit medical, agricultural, and industrial applications. Genetic engineering has led to the development of genetically modified organisms (GMOs), gene therapies, and advanced vaccines that have revolutionized modern medicine and food production.
2. Bioprocess Engineering – This branch of biotechnology involves the application of chemical engineering principles to optimize biological processes. It focuses on maintaining a controlled and sterile environment for the large-scale growth of microbes or eukaryotic cells, ensuring the efficient production of biotechnological products. This field is essential for the industrial-scale production of pharmaceuticals, including insulin, monoclonal antibodies, antibiotics, and vaccines. Bioprocess engineering also contributes to the production of biofuels, biodegradable plastics, and other sustainable bioproducts that promote environmental sustainability.
Biotechnology continues to evolve, offering groundbreaking solutions to global challenges such as disease treatment, food security, and environmental protection. As new innovations emerge, the field holds immense potential to improve human health, enhance agricultural productivity, and develop sustainable industrial processes for a better future.
BASICS OF GENETICS
GENE
A gene is a segment of DNA that contains the instructions necessary to create a functional product, typically a protein. Genes are the fundamental units of heredity and play a crucial role in determining an organism’s traits. The human genome is estimated to contain between 20,000 and 25,000 genes, each responsible for different biological functions.
Genes can be classified into three main categories based on their function:
1. Structural Genes – These genes contain the coding sequences needed to produce proteins. Proteins are essential for various cellular processes, including metabolism, cell signaling, and structural support.
2. Regulatory Genes – These genes control the expression of other genes by turning them on or off when needed. They play a vital role in development, differentiation, and response to environmental stimuli. Regulatory genes ensure that proteins are produced at the right time, in the right amount, and in the appropriate cells.
3. Non-Coding Genes – While these genes do not code for proteins, they perform important regulatory functions. Many non-coding genes produce RNA molecules, such as microRNAs or long non-coding RNAs, which help regulate gene expression and maintain genome stability.
Understanding genes and their functions is essential in fields like genetics, biotechnology, and medicine. Advances in genetic research have led to breakthroughs in disease treatment, gene therapy, and personalized medicine, revolutionizing healthcare and improving lives.
DNA
All known living organisms store their genetic instructions for growth, development, and reproduction in a molecule called deoxyribonucleic acid (DNA). This remarkable molecule carries the hereditary information that determines an organism’s traits, from the simplest bacteria to complex human beings. While most living organisms rely on DNA for genetic storage, some viruses use RNA (ribonucleic acid) instead.
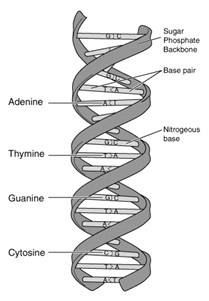
Structure of DNA
DNA is composed of four fundamental nucleotide bases that form the language of genetic instructions:
- Adenine (A)
- Thymine (T)
- Cytosine (C)
- Guanine (G)
DNA is structured as a double helix, consisting of two complementary strands twisted around each other. Each strand is made up of a sequence of nucleotides, and the specific order of these bases encodes genetic information.
DNA Replication and Base Pairing
One of DNA’s most critical functions is its ability to replicate accurately, ensuring that genetic information is faithfully passed on from one generation to the next. This is made possible by base pairing rules, where:
- Adenine (A) always pairs with Thymine (T)
- Cytosine (C) always pairs with Guanine (G)
During DNA replication, the double helix unwinds, and each strand serves as a template for forming a new complementary strand, maintaining genetic integrity.
The Role of DNA in Protein Synthesis
DNA plays a fundamental role in the synthesis of proteins, which are essential for cellular functions, tissue development, and overall organismal growth. Through the processes of transcription and translation, DNA provides the instructions for building proteins that regulate metabolism, immune responses, and other biological activities.
Importance of DNA Research
Advances in DNA research have revolutionized genetics, biotechnology, and medicine. Innovations such as DNA fingerprinting, genetic engineering, gene therapy, and genome sequencing have transformed healthcare, forensic science, and agriculture. Understanding DNA is crucial for unlocking the mysteries of life, diagnosing genetic disorders, and developing cutting-edge treatments to improve human health.
CHROMOSOMES
The majority of living cells contain chromosomes, which are thread-like structures composed of DNA and proteins. Chromosomes serve as the primary storage units for genetic information, carrying genes that determine an organism’s inherited traits. These genes dictate everything from physical appearance to biological functions, ensuring that genetic characteristics are passed from one generation to the next.
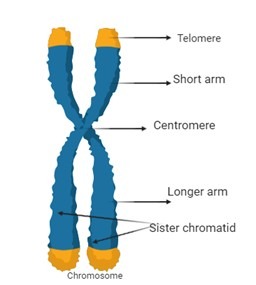
Structure and Function of Chromosomes
Chromosomes are made up of tightly coiled DNA wrapped around histone proteins, allowing them to fit within the nucleus of a cell. This compact structure helps in the proper organization, replication, and distribution of genetic material during cell division. Each chromosome contains numerous genes, which act as instructions for making proteins that control various biological processes essential for life.
Chromosome Numbers in Different Species
The number of chromosomes varies among different species, with each species having a specific chromosome count. For example:
- Humans have 46 chromosomes, arranged in 23 pairs, with one set inherited from each parent.
- Dogs have 78 chromosomes, arranged in 39 pairs.
- Fruit flies (Drosophila melanogaster) have only 8 chromosomes, arranged in 4 pairs.
In humans, 22 pairs of chromosomes are called autosomes, which determine most of an individual’s traits, while the 23rd pair consists of sex chromosomes (XX in females and XY in males), which determine biological sex.
The Role of Chromosomes in Cell Division
Chromosomes play a crucial role in cell division, ensuring the accurate transfer of genetic material to new cells. There are two main types of cell division:
- Mitosis, which produces genetically identical cells for growth, repair, and maintenance.
- Meiosis, which occurs in reproductive cells and results in the formation of gametes (sperm and egg cells) with half the chromosome number, enabling genetic diversity in offspring.
Chromosome Abnormalities and Their Impact
Any changes in chromosome number or structure can lead to genetic disorders. For example:
- Down syndrome occurs when an individual has an extra copy of chromosome 21 (trisomy 21).
- Turner syndrome results from the absence of one X chromosome in females.
- Klinefelter syndrome occurs in males with an extra X chromosome (XXY).
Importance of Chromosome Research
Understanding chromosomes has led to significant advancements in genetics, medicine, and biotechnology. Chromosomal studies help in diagnosing genetic disorders, improving fertility treatments, and developing targeted therapies for various diseases. Researchers continue to explore the role of chromosomes in evolution, heredity, and human health, leading to new breakthroughs in science and medicine.
RNA
Ribonucleic acid (RNA) is a single-stranded molecule that plays a crucial role in various biological functions, particularly in protein synthesis and the transfer of genetic information. RNA serves as an intermediary between DNA and proteins, helping cells interpret genetic instructions and carry out essential processes.
Structure and Composition of RNA
Although RNA shares some similarities with DNA, it differs in several key ways:
- RNA is single-stranded, whereas DNA is double-stranded.
- RNA contains ribose sugar, while DNA contains deoxyribose sugar (which lacks one oxygen atom).
- RNA uses the nitrogenous base uracil (U) instead of thymine (T), pairing with adenine (A) in genetic sequences.
The four nitrogenous bases found in RNA are:
- Adenine (A)
- Cytosine (C)
- Uracil (U) (unique to RNA)
- Guanine (G)
Types of RNA and Their Functions
RNA is a versatile molecule with different types, each performing specialized functions:
- Messenger RNA (mRNA) – Acts as a blueprint for protein synthesis by carrying genetic instructions from DNA in the nucleus to ribosomes in the cytoplasm.
- Ribosomal RNA (rRNA) – A structural component of ribosomes, the cellular machinery responsible for assembling proteins.
- Transfer RNA (tRNA) – Delivers specific amino acids to ribosomes during protein synthesis, ensuring the correct sequence for protein formation.
- MicroRNA (miRNA) and Small Interfering RNA (siRNA) – Play regulatory roles in gene expression by silencing or modulating specific genes, helping control cell processes.
RNA’s Role in Protein Synthesis
RNA plays a fundamental role in gene expression and protein production through two main processes:
- Transcription – DNA is copied into mRNA, which carries genetic instructions to the ribosomes.
- Translation – rRNA and tRNA help decode mRNA instructions to assemble amino acids into proteins.
RNA in Research and Medicine
RNA research has led to groundbreaking advancements in genetics, molecular biology, and medicine. RNA-based technologies have been instrumental in developing:
- mRNA vaccines, such as COVID-19 vaccines, which use mRNA to instruct cells to produce an immune response.
- RNA interference (RNAi) therapies, which target and silence disease-causing genes.
- Gene expression studies, helping scientists understand diseases and develop new treatments.
RNA continues to be a key focus in biotechnology and medical research, offering promising solutions for treating genetic disorders, cancer, and viral infections. Understanding RNA’s structure and functions provides critical insights into life’s molecular mechanisms and its potential applications in science and medicine.
RNA vs DNA
RNA (Ribonucleic Acid) and DNA (Deoxyribonucleic Acid) are both essential nucleic acids that carry and transmit genetic information. While they share similarities, they differ significantly in structure, function, and stability, allowing them to play distinct roles in cellular processes.
1. Structure
- DNA: A double-stranded molecule forming a double helix, providing a stable storage system for genetic information.
- RNA: A single-stranded molecule that can fold into complex secondary structures, enabling it to perform various biological functions.
2. Sugar Composition
- DNA: Contains deoxyribose, a sugar that lacks an oxygen atom at the 2′ carbon, making DNA more stable.
- RNA: Contains ribose, which has an extra hydroxyl (-OH) group at the 2′ carbon, making RNA more reactive and less stable.
3. Nitrogenous Bases
- DNA: Uses thymine (T), which pairs with adenine (A) during base pairing.
- RNA: Uses uracil (U) instead of thymine (T) but still pairs with adenine (A).
4. Function
- DNA: Serves as the permanent blueprint for storing genetic information and passing it from one generation to the next.
- RNA: Plays an active role in protein synthesis, gene regulation, and cellular functions, acting as a messenger and catalyst in biological processes.
5. Stability
- DNA: More chemically stable, thanks to its double-stranded structure and the absence of a reactive hydroxyl group, making it suitable for long-term genetic storage.
- RNA: Less stable and more prone to degradation due to its single-stranded nature and extra hydroxyl group, allowing for temporary gene expression and rapid cellular responses.
6. Types of RNA vs. DNA
- DNA: Exists primarily as a single type that stores genetic information.
- RNA: Exists in multiple forms, each with specialized functions:
- mRNA (messenger RNA): Carries genetic instructions from DNA to ribosomes for protein synthesis.
- tRNA (transfer RNA): Helps assemble amino acids into proteins during translation.
- rRNA (ribosomal RNA): Forms the core structural and functional components of ribosomes, where proteins are synthesized.
- Other types: Small RNAs like microRNA (miRNA) and small interfering RNA (siRNA) help regulate gene expression.
7. Location in the Cell
- DNA: Found primarily in the nucleus of eukaryotic cells (with small amounts in mitochondria and chloroplasts).
- RNA: Found in both the nucleus and cytoplasm, where it plays an active role in protein synthesis and cellular regulation.
The Significance of RNA and DNA
DNA serves as the foundation for heredity, ensuring genetic information is preserved and transmitted across generations. RNA, on the other hand, acts as a dynamic molecule that helps interpret and execute the instructions stored in DNA. The interplay between DNA and RNA is fundamental to life, influencing everything from genetic expression to modern medical advancements such as mRNA vaccines and gene therapies.
PROTEIN SYNTHESIS
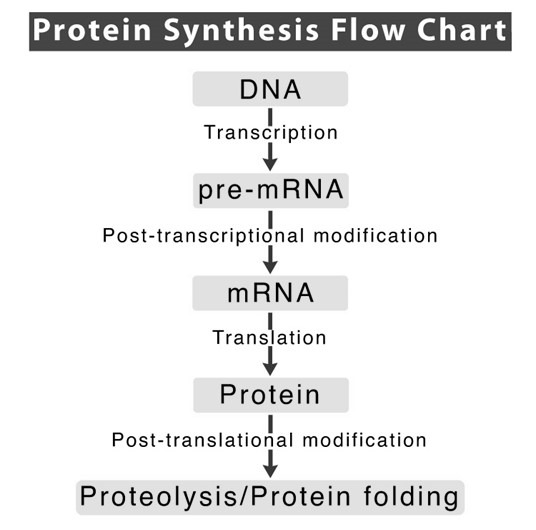
The central dogma of molecular biology describes the flow of genetic information in a cell, following the sequence:
DNA → RNA → Proteins
This process ensures that genetic instructions stored in DNA are accurately transferred and used to produce proteins, which are essential for cell structure, function, and regulation. The three main stages of this process are DNA replication, transcription, and translation.
1. DNA Replication: Copying Genetic Information
DNA replication is the process by which a cell makes an identical copy of its DNA before cell division. This ensures that each new cell receives the same genetic instructions. During replication:
- The double helix unwinds, and the two strands separate.
- Each strand serves as a template for the creation of a new complementary strand.
- Special enzymes like DNA polymerase add new nucleotides following base-pairing rules (A pairs with T, and C pairs with G).
- The result is two identical DNA molecules, each containing one original strand and one newly synthesized strand (semi-conservative replication).
2. Transcription: Converting DNA into RNA
Transcription is the process where a segment of DNA is copied into messenger RNA (mRNA), which carries genetic instructions from the nucleus to the cytoplasm.
Steps of Transcription:
- Initiation: The enzyme RNA polymerase binds to a specific DNA region called the promoter, signaling the start of transcription.
- Elongation: RNA polymerase reads the DNA template and assembles a complementary RNA strand, replacing thymine (T) with uracil (U).
- Termination: Once the RNA strand is complete, it detaches from the DNA, and the newly formed mRNA is processed and transported to the cytoplasm.
mRNA serves as a blueprint for protein synthesis, carrying genetic instructions for assembling amino acids into proteins.
3. Translation: Synthesizing Proteins from RNA
Translation is the process where the genetic code in mRNA is used to assemble amino acids into proteins. It occurs on ribosomes in the cytoplasm and involves multiple types of RNA:
- mRNA carries genetic instructions.
- tRNA (transfer RNA) delivers the correct amino acids.
- rRNA (ribosomal RNA) helps form ribosomes, the site of protein synthesis.
Steps of Translation:
1. Initiation: The ribosome binds to the mRNA, and a start codon (AUG) signals the beginning of translation.
2. Elongation:
- tRNA molecules bring amino acids matching the codons in mRNA.
- Amino acids are linked together, forming a polypeptide chain.
3. Termination: When the ribosome reaches a stop codon (UAA, UAG, or UGA), translation ends, and the completed protein is released.
The newly formed protein undergoes folding and modifications to become functional, contributing to various cellular activities.
The Importance of Genetic Information Transfer
The accurate transfer of genetic information is essential for:
✔ Cell growth and repair – ensuring that new cells function correctly.
✔ Enzyme and hormone production – necessary for metabolism and biological regulation.
✔ Genetic inheritance – allowing traits to be passed from parents to offspring.
✔ Biotechnology and medicine – understanding gene expression has led to advances in gene therapy, genetic engineering, and mRNA vaccines.
By decoding the processes of DNA replication, transcription, and translation, scientists continue to make breakthroughs in genetics, medicine, and biotechnology, improving treatments for diseases and advancing our understanding of life.
Dark DNA
Dark DNA refers to regions of the genome that are difficult to detect or analyze using traditional sequencing techniques. These regions often contain highly repetitive sequences, mutations, or structural variations, making them elusive in genetic research. Despite being overlooked for years, recent discoveries suggest that dark DNA plays a crucial role in gene regulation, evolution, and species adaptation.
Why Is Dark DNA Hard to Detect?
Genomic sequencing technologies rely on assembling short DNA fragments into a complete genome. However, certain characteristics of dark DNA make it difficult to sequence accurately:
- Repetitive Sequences: Some DNA regions contain repeating patterns, confusing sequencing algorithms and making it challenging to determine their exact order.
- High Mutation Rates: Frequent mutations can create variations that evade detection using standard sequencing methods.
- Structural Complexity: Some genes may be hidden in highly compacted or structurally unique regions of the genome.
Due to these challenges, large portions of dark DNA remain uncharacterized, leading scientists to explore new techniques to uncover its full potential.
The Role of Dark DNA in Genetics
Although once dismissed as “junk DNA,” dark DNA is now believed to play an essential role in several biological processes, including:
1. Gene Regulation:
- Dark DNA may contain regulatory elements that control when and how genes are turned on or off.
- It could influence RNA processing, chromatin structure, and transcription factor binding.
2. Evolution and Adaptation:
- Some species have developed unique adaptations due to hidden genetic sequences within dark DNA.
- It may provide a reservoir for genetic diversity, allowing organisms to evolve and survive in changing environments.
3. Disease and Genetic Disorders:
- Researchers suspect that mutations in dark DNA regions may contribute to neurological disorders, cancers, and inherited diseases.
- Understanding dark DNA could lead to new diagnostic tools and treatments for genetic conditions.
Unlocking the Secrets of Dark DNA
With advances in genome sequencing and artificial intelligence, scientists are beginning to uncover dark DNA’s hidden functions. Long-read sequencing and epigenetic mapping are improving our ability to explore these mysterious genetic regions.
The study of dark DNA could lead to groundbreaking discoveries in genetics, medicine, and evolutionary biology. As research continues, this once-overlooked portion of the genome may hold the key to understanding genetic complexity, disease mechanisms, and the evolution of life itself.
GENOME SEQUENCING
Genome sequencing is the process of determining the entire DNA sequence of an organism’s genome, identifying the precise order of the four nucleotide bases—adenine (A), thymine (T), cytosine (C), and guanine (G)—across all chromosomes. This revolutionary technique has transformed genetics and biotechnology, allowing scientists to explore genetic variations, understand inherited traits, detect mutations linked to diseases, and develop groundbreaking advancements in personalized medicine and biotechnology.
Major Genome Sequencing Projects
Human Genome Project (HGP)
The idea of sequencing all 3.2 billion nucleotide pairs in the human genome was first proposed in the 1980s. In 1990, the Human Genome Project (HGP) was launched, with the ambitious goal of mapping every human gene, creating a comprehensive physical map of the entire genome, and determining the complete nucleotide sequence of all 24 human chromosomes by 2005.
This landmark project revolutionized genetic research, leading to new discoveries in disease genetics, gene therapy, and biotechnology while advancing the field of personalized medicine.
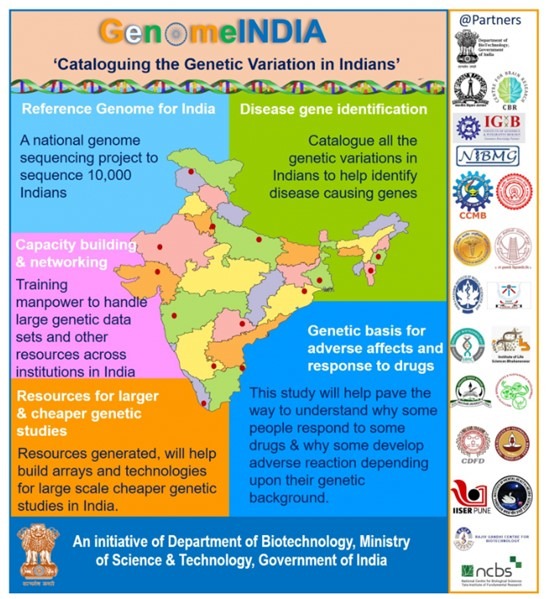
Genome India Project (GIP)
Launched in 2020 by the Department of Biotechnology (DBT), the Genome India Project aims to sequence the genomes of 10,000 individuals from diverse ethnic and geographical backgrounds across India.
- The project seeks to build a comprehensive genetic database that will help in understanding disease patterns, improving precision medicine, and developing targeted preventive care and diagnostics.
- Led by the Centre for Brain Research at IISc, Bengaluru, in collaboration with 20 universities, this initiative strengthens India’s capabilities in genomic research and next-generation healthcare.
Human Microbiome Initiative of Select Endogamous Population of India
This research initiative focuses on studying the microorganisms associated with human populations in various endogamous communities, including indigenous groups with limited exposure to modern lifestyles.
Using metagenomic techniques, the study explores how factors such as age, geography, diet, and lifestyle shape the gut microbiome. Additionally, it examines potential links between microbial enterotypes and the three Ayurvedic Prakriti types, offering deeper insights into the microbiome’s role in human health and disease.
Earth Bio-Genome Project (EBP)
The Earth Bio-Genome Project (EBP) is a global initiative aimed at sequencing and analyzing the genomes of all eukaryotic life on Earth to create a comprehensive genetic library for biodiversity conservation and sustainability.
- Over a 10-year period, the project aims to sequence 1.5 million species in three phases.
- The EBP project will establish an accurate genetic reference for understanding evolutionary relationships among various species, forming the foundation for a “Digital Library of Life.”
This ambitious effort will enhance conservation efforts, support agriculture and biotechnology, and provide solutions for global environmental challenges.
INDIgen Project
The INDIgen Project was initiated by CSIR (Council of Scientific and Industrial Research) to sequence the entire genomes of thousands of Indian individuals, creating a diverse and extensive genetic database.
- The project focuses on understanding India’s vast genetic diversity and its implications for health, disease susceptibility, and drug response.
- By studying this data, researchers aim to enhance personalized medicine, predictive diagnostics, and precision healthcare tailored specifically for the Indian population.
Somatic Cell Nuclear Transfer (SCNT): Cloning and Its Applications
Somatic Cell Nuclear Transfer (SCNT) is a powerful laboratory technique in developmental biology and genetics used to create an embryo from a somatic (body) cell and an egg cell.
Process of SCNT
- Somatic Cell Extraction: A body cell is removed from the organism to be cloned. Since somatic cells contain the complete genetic information of the organism, they serve as the genetic donor.
- Enucleation of Egg Cell: An egg cell is extracted from a female donor, and its nucleus (genetic material) is removed, creating an enucleated egg.
- Nuclear Transfer: The nucleus from the somatic cell is introduced into the enucleated egg, ensuring that the egg now contains the genetic material of the donor organism.
- Activation and Development: The reconstructed egg is chemically or electrically stimulated, initiating cell division. The developing embryo forms a blastocyst, an early-stage embryo.
Applications of SCNT
- Cloning: The most well-known application of SCNT is the cloning of organisms. Dolly the sheep, created in 1996, was the first mammal cloned from an adult somatic cell.
- Therapeutic Cloning: SCNT is used to generate genetically identical stem cells for treating diseases, studying genetic disorders, and advancing regenerative medicine.
- Conservation: Scientists explore SCNT as a method for cloning endangered species, helping preserve genetic diversity and prevent species extinction.
Ethical and Scientific Considerations
Despite its potential, SCNT raises ethical concerns, particularly regarding animal welfare and the implications of human cloning. Additionally, SCNT has a low success rate, with many cloned embryos experiencing abnormal development, making the process inefficient.
The Future of Genome Sequencing and Genetic Research
With advances in genome sequencing technologies, researchers are uncovering new insights into human health, evolution, and biodiversity. From personalized medicine to conservation genomics, genome sequencing continues to revolutionize science and medicine. As technology improves, these pioneering projects will help shape the future of biotechnology, healthcare, and environmental sustainability, opening new doors for scientific discovery and innovation.
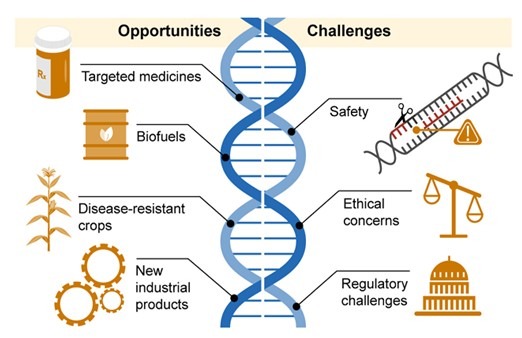
GENOME EDITING
Genome editing, also known as genome engineering or gene editing, is a powerful biotechnological tool that enables scientists to add, remove, modify, or replace DNA within an organism’s genome. These techniques have revolutionized genetic research, allowing precise modifications to correct genetic disorders, enhance agricultural crops, and study gene functions in various organisms.
Applications of Genome Editing
- Medical Advancements: Used to develop therapies for genetic disorders such as sickle cell anemia, cystic fibrosis, and certain cancers.
- Agricultural Improvements: Enhances crop resistance to pests, diseases, and environmental stress, improving food security.
- Biotechnology and Research: Helps in drug discovery, disease modeling, and understanding fundamental biological processes.
Genome Editing Techniques
Several advanced techniques have been developed for genome editing, each with unique mechanisms and applications:
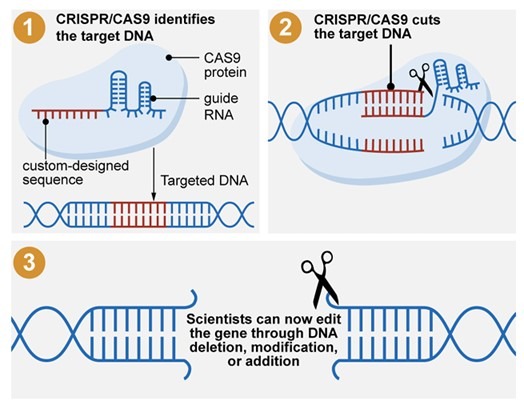
1. CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-Associated Protein 9)
- CRISPR-Cas9 is the most widely used and revolutionary genome-editing tool due to its simplicity, precision, and efficiency.
How CRISPR-Cas9 Works:
- The system consists of a guide RNA (gRNA) that binds to a specific DNA sequence through complementary base pairing.
- The Cas9 enzyme, a nuclease, then cuts the targeted DNA at the desired location, enabling modifications such as gene deletion, insertion, or correction.
Discovery and Nobel Prize Recognition
- Emmanuelle Charpentier and Jennifer A. Doudna discovered the CRISPR-Cas9 mechanism and were awarded the 2020 Nobel Prize in Chemistry for their groundbreaking work.
- CRISPR technology is now being explored for treating genetic disorders, infectious diseases, and even cancer therapy.
2. TALENs (Transcription Activator-Like Effector Nucleases)
- TALENs are engineered enzymes used for precise gene editing. They consist of:
- A DNA-binding domain derived from transcription activator-like effectors (TALEs), which recognize specific DNA sequences.
- A nuclease domain that cuts DNA at the targeted site, allowing modifications to be introduced.
Advantages of TALENs:
- Highly specific and customizable for targeting various genes.
- Effective for genome modifications in plants, animals, and human cells.
3. Zinc-Finger Nucleases (ZFNs)
ZFNs are artificial restriction enzymes engineered by fusing:
- A zinc-finger DNA-binding domain, which can recognize and bind specific DNA sequences.
- The FokI restriction endonuclease cleavage domain, which cuts the DNA at the targeted site.
How ZFNs Work:
- The FokI cleavage domain requires two flanking zinc-finger binding sites separated by a spacer sequence of 5–7 base pairs to efficiently break DNA.
- The DNA break triggers the cell’s natural repair mechanisms, allowing for gene modifications.
Applications of ZFNs:
- Used in gene therapy for diseases like HIV and sickle cell anemia.
- Applied in plant biotechnology for crop improvement.
4. Homing Endonucleases (Meganucleases)
Homing endonucleases, also called meganucleases, are highly specific enzymes that:
- Recognize and cut long DNA sequences (typically 14–40 base pairs).
- Have a sequence-specific binding mechanism for precise DNA cleavage.
Key Differences from Other Gene Editing Tools:
- Unlike ZFNs and TALENs, homing endonucleases do not have modular binding and cleavage domains.
- They interact extensively with their DNA substrate, making them highly specific but less flexible compared to CRISPR and other methods.
The Future of Genome Editing
Genome editing continues to advance, with researchers developing newer, more efficient techniques to enhance precision, minimize off-target effects, and expand its applications in medicine, agriculture, and biotechnology.
- Gene Therapy: Genome editing holds promise for treating genetic disorders by correcting defective genes at the DNA level.
- Personalized Medicine: CRISPR and other techniques may help tailor treatments based on an individual’s genetic profile.
- Environmental and Agricultural Benefits: Genome editing can produce disease-resistant crops, reduce the need for pesticides, and create sustainable agricultural solutions.
As genome editing technologies evolve, ethical considerations regarding human gene modification, biodiversity conservation, and genetic privacy must also be addressed. However, its potential to redefine medicine, science, and agriculture makes it one of the most groundbreaking advancements in modern biotechnology.
RNA INTERFERENCE (RNAI)
RNA interference (RNAi) is a natural cellular process that plays a crucial role in gene regulation and defense against viruses. This mechanism silences specific genes, preventing them from producing proteins. Scientists have harnessed RNAi technology for various applications in medical research, agriculture, and drug development.
How RNA Interference Works
RNAi is a highly precise mechanism that relies on small RNA molecules to target and degrade specific messenger RNA (mRNA), thereby preventing protein synthesis. The process occurs in several steps:
1. Triggering RNAi
RNA interference is initiated when double-stranded RNA (dsRNA) enters the cell. This dsRNA can originate from:
- Viruses that introduce foreign genetic material into the host cell.
- Endogenous sources, where cells naturally produce regulatory RNAs.
- Experimental introduction, where scientists artificially introduce dsRNA to silence specific genes for research or therapeutic purposes.
2. RNA Chopping: Formation of Small Interfering RNAs (siRNA)
- The enzyme Dicer processes the dsRNA into short fragments called small interfering RNAs (siRNAs).
- These siRNAs are typically 21–23 nucleotides long and serve as guide molecules for silencing specific genes.
3. RISC Complex Formation
- The RNA-induced silencing complex (RISC) binds to the siRNA fragments.
- One strand of the siRNA is removed, while the remaining strand serves as a guide to target specific mRNA molecules.
4. Targeting and Silencing the Gene
- The RISC-siRNA complex binds to its corresponding mRNA, recognizing it through complementary base pairing.
- Once bound, RISC cleaves the mRNA, leading to its degradation.
- Since mRNA is essential for protein synthesis, its destruction prevents the production of the target protein, effectively silencing the gene.
This gene-silencing process helps regulate cellular protein production and provides a defense mechanism against viruses that introduce harmful genetic material into cells.
Applications of RNA Interference (RNAi)
1. Scientific Research
- RNAi is an essential tool in functional genomics, allowing scientists to study the role of specific genes.
- By selectively silencing genes, researchers can determine their biological functions and interactions.
- Used in disease modeling, where genes associated with disorders are silenced to understand their impact.
2. Medical Applications: RNAi-Based Drugs
RNAi technology is being used to develop novel therapeutic treatments by targeting and silencing disease-causing genes. Some key medical applications include:
- Cancer Therapy: RNAi is being explored to silence oncogenes (genes that cause cancer), preventing tumor growth.
- Genetic Disorders: Used to treat genetic diseases by shutting down faulty genes responsible for conditions like Huntington’s disease and amyloidosis.
- Viral Infections: RNAi-based treatments are being developed to combat viruses like Hepatitis B, HIV, and SARS-CoV-2 by disrupting viral replication.
Several RNAi-based drugs have already been approved or are in clinical trials, marking a significant advancement in gene therapy and precision medicine.
3. Agriculture: Pest-Resistant Crops
RNA interference is revolutionizing agriculture by creating crops with enhanced resistance to pests and diseases.
Case Study: Tobacco Plant Protection Against Nematodes
- The root-knot nematode (Meloidogyne incognita) is a parasitic worm that infects plant roots, reducing crop yield.
- Scientists used RNAi technology to introduce nematode-specific genes into tobacco plants using Agrobacterium vectors.
- The transgenic plants expressed interfering RNA, which silenced essential genes in the nematodes.
- As a result, the parasites were unable to survive, protecting the crops from infestation.
Similar RNAi-based techniques are being explored to protect other crops like corn, soybeans, and cotton from pests, reducing the need for chemical pesticides and promoting sustainable agriculture.
The Future of RNA Interference (RNAi)
RNAi has transformed biotechnology by providing a powerful and precise method for gene regulation. Future developments in RNAi technology will likely focus on:
- Developing more RNAi-based drugs for complex diseases.
- Expanding agricultural applications to improve crop resilience against climate change.
- Enhancing delivery methods to ensure RNAi molecules reach target cells effectively in humans.
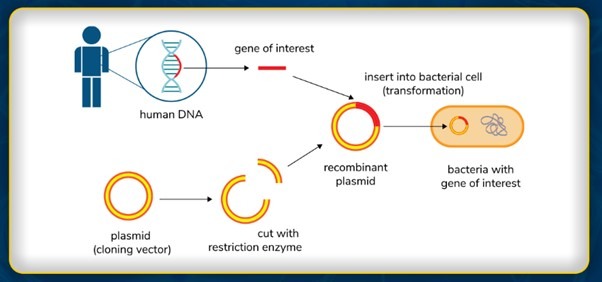
RECOMBINANT DNA TECHNOLOGY
Recombinant DNA technology is a groundbreaking method of genetic engineering that allows scientists to modify an organism’s genetic composition by introducing DNA from multiple sources. This technique is widely used in medicine, agriculture, and biotechnology to create genetically modified organisms (GMOs), develop life-saving drugs, and enhance agricultural productivity.
What is Recombinant DNA?
Recombinant DNA (rDNA) is an artificially created DNA molecule that combines genetic material from two or more sources. It is produced using laboratory techniques, enabling the modification, insertion, or deletion of genes to achieve desired traits in an organism.
This technology is essential for gene therapy, protein production, and the development of genetically modified crops that are resistant to pests and environmental stress.
Steps in Recombinant DNA Technology
1. Restriction Enzymes: Cutting DNA at Specific Sites
- Specialized enzymes called restriction endonucleases recognize specific DNA sequences and cut the DNA at these sites.
- This produces DNA fragments with “sticky ends” that can easily bind to complementary sequences from different DNA sources.
- The cut DNA is then mixed with foreign DNA (from another organism) to create a recombinant DNA molecule.
2. Selectable Markers and Cloning Sites
- Selectable markers help in identifying successful recombinants (cells that have successfully incorporated the foreign DNA).
- Cloning sites provide a location where foreign DNA can be inserted within the host genome.
3. Techniques for Inserting DNA into Host Cells
Different methods are used to introduce recombinant DNA into host cells:
A. Microinjection (For Animal Cells)
- DNA is directly injected into the nucleus of animal cells using a fine needle.
- This technique is commonly used in transgenic animal research and gene therapy.
B. Biolistics (For Plant Cells)
- Also known as the gene gun method, this technique bombards plant cells with metal particles coated with foreign DNA.
- The plant cells integrate the new DNA, leading to the development of genetically modified crops.
C. Polymerase Chain Reaction (PCR) – Amplifying Target Genes
- PCR technology allows scientists to amplify specific DNA segments, creating millions of copies of the desired gene.
- This ensures an adequate amount of DNA for successful cloning and genetic modification.
4. Cloning Vectors: Vehicles for Introducing DNA into Host Cells
Vectors serve as carriers that transport recombinant DNA into host cells. Some of the most commonly used vectors include:
- Plasmids: Circular DNA molecules used in bacteria for gene transfer.
- Bacteriophages: Viruses that infect bacteria, used for large-scale DNA cloning.
- Cosmids and Artificial Chromosomes: Engineered for larger DNA fragments, used in genome research.
5. Key Components of Cloning Vectors
- Origin of Replication (Ori): Ensures that the recombinant DNA is copied and propagated inside the host cell.
- Promoter Sequences: Allow the desired gene to be expressed, leading to protein production.
6. Gene Expression: Producing Functional Proteins
Once the recombinant DNA is inside the host cell, the cell begins expressing the introduced gene. This results in:
- The production of beneficial proteins (e.g., insulin, enzymes, hormones).
- Genetically modified organisms (GMOs) with enhanced traits such as pest resistance in plants.
Applications of Recombinant DNA Technology
1. Medicine: Developing Life-Saving Drugs and Therapies
- Insulin Production: rDNA technology is used to produce human insulin for diabetes treatment.
- Vaccine Development: Hepatitis B and COVID-19 vaccines are created using recombinant DNA techniques.
- Gene Therapy: Scientists use rDNA to correct genetic disorders, such as cystic fibrosis and hemophilia.
- Monoclonal Antibodies: Used in cancer therapy and autoimmune disease treatment.
2. Agriculture: Genetically Modified Crops (GMOs)
- Pest-Resistant Crops: Plants like Bt cotton and Bt corn are engineered to produce their own insecticides, reducing the need for chemical pesticides.
- Drought-Resistant Crops: Genetic modifications improve crop survival in water-scarce environments.
- Nutrient-Enriched Crops: Golden rice, enhanced with vitamin A, helps combat malnutrition.
3. Research and Biotechnology
- Functional Genomics: Understanding gene functions by silencing or modifying genes.
- Stem Cell Research: Producing genetically engineered stem cells for regenerative medicine.
- Synthetic Biology: Creating artificial genes and organisms for industrial applications.
Future of Recombinant DNA Technology
The potential of recombinant DNA technology continues to expand, with new research focusing on:
- Personalized Medicine: Tailoring drug treatments based on an individual’s genetic makeup.
- Sustainable Agriculture: Developing crops that require fewer resources while producing higher yields.
- Bioengineered Organs: Using genetically modified cells to grow human organs for transplantation.
THREE PARENT BABY/ MITOCHONDRIAL REPLACEMENT THERAPY
Mitochondrial Replacement Therapy (MRT), also known as the “three-parent baby” technique, is an advanced reproductive technology designed to prevent the transmission of mitochondrial diseases from mother to child. It involves the combination of genetic material from three individuals—the biological parents and a mitochondrial donor—through in vitro fertilization (IVF). This groundbreaking method allows couples at risk of passing on mitochondrial disorders to conceive a genetically related child with healthy mitochondria.
Understanding Mitochondrial Diseases
Mitochondria, often referred to as the powerhouses of the cell, are responsible for generating energy necessary for cellular functions. They contain their own small circular DNA, distinct from the nuclear DNA inherited from both parents. Mitochondrial disorders occur when mutations in mitochondrial DNA impair the ability to produce energy efficiently.
Symptoms of Mitochondrial Diseases
Mitochondrial disorders can affect multiple organs and lead to:
- Neurological issues (seizures, developmental delays)
- Muscle weakness and fatigue
- Heart and liver dysfunction
- Hearing and vision impairment
- Metabolic disorders
Since mitochondria are inherited exclusively from the mother, any defect in her mitochondrial DNA can be passed down to her children. MRT prevents the transmission of these disorders by replacing defective mitochondria with healthy donor mitochondria.
How Mitochondrial Replacement Therapy Works
MRT is performed during in vitro fertilization (IVF) and involves transferring nuclear genetic material from the intended parents into an egg with healthy mitochondria from a donor.
Mechanism of Mitochondrial Replacement
- The nuclear genetic material from the mother’s egg is removed, leaving behind only the defective mitochondria.
- This nucleus is then transferred into a donor egg that has had its own nuclear genetic material removed but retains its healthy mitochondria.
- The reconstructed egg is fertilized with sperm from the biological father and implanted into the mother’s uterus, leading to a genetically related child free of mitochondrial disease.
Since the child inherits nuclear DNA from both biological parents (which determines traits such as appearance and intelligence) and mitochondrial DNA from the donor, it is sometimes referred to as a “three-parent baby”—though the donor contributes less than 1% of the child’s genetic makeup.
Techniques of Mitochondrial Replacement Therapy
There are two main approaches used in MRT:
1. Pronuclear Transfer (PNT)
- Involves fertilizing both the mother’s egg and the donor’s egg with the father’s sperm.
- The pronuclei (structures that contain genetic material) from both fertilized eggs are removed.
- The mother’s pronucleus is transferred into the donor’s fertilized egg, which contains healthy mitochondria.
- The reconstructed embryo is then implanted into the mother’s uterus.
2. Maternal Spindle Transfer (MST)
- The nuclear DNA from the mother’s unfertilized egg is extracted, leaving behind her defective mitochondria.
- This nuclear DNA is then inserted into a donor egg with healthy mitochondria (after removing the donor’s nucleus).
- The modified egg is fertilized with the father’s sperm and implanted into the mother’s uterus.
Benefits of Mitochondrial Replacement Therapy
- Prevents the transmission of mitochondrial diseases, ensuring a healthy child.
- Allows couples with a history of mitochondrial disorders to conceive a biologically related child.
- Enhances IVF success rates by improving egg health.
- Opens possibilities for treating infertility in older women, as aging affects mitochondrial function.
STEM CELL THERAPY
Stem cell therapy, also known as regenerative medicine, is an advanced medical approach that utilizes stem cells or their derivatives to stimulate the body’s natural repair mechanisms. It aims to restore, repair, or replace damaged, diseased, or dysfunctional tissues, offering revolutionary treatments for conditions that were once considered untreatable.
Stem cells possess the unique ability to self-renew and differentiate into specialized cell types, such as heart muscle cells, blood cells, or nerve cells. This characteristic makes them a powerful tool in modern medicine for treating degenerative diseases, injuries, and organ damage.
Sources of Stem Cells
Stem cells can be derived from various sources, broadly classified into prenatal, adult, and induced pluripotent stem cells (iPSCs).
1. Prenatal Stem Cells (Embryonic and Extra-Embryonic Sources)
These stem cells originate from the earliest stages of human development and include:
- Embryonic Stem Cells (ESCs): Derived from the blastocyst stage of an embryo, they are pluripotent, meaning they can develop into any type of cell in the body.
- Placental Stem Cells: Found in the placenta, these cells are rich in regenerative properties and are collected after birth without harming the baby or mother.
- Umbilical Cord Blood Stem Cells: Collected from umbilical cord blood, these cells are particularly useful in treating blood disorders, immune deficiencies, and metabolic conditions.
- Amniotic Fluid Stem Cells: Found in the amniotic sac, these stem cells hold promise for regenerative applications in bone, muscle, and organ repair.
2. Adult Stem Cells (Tissue-Specific or Somatic Stem Cells)
Adult stem cells are found in various tissues and organs of the body and help with natural tissue repair. Unlike embryonic stem cells, they are multipotent, meaning they can develop into a limited number of specialized cell types. Common sources include:
- Bone Marrow: Rich in hematopoietic stem cells (HSCs), which are used in bone marrow transplants to treat leukemia and blood disorders.
- Adipose (Fat) Tissue: Contains mesenchymal stem cells (MSCs), which can develop into bone, cartilage, and muscle cells, making them valuable in regenerative medicine.
- Skeletal Muscle: Contains muscle stem cells (satellite cells) that assist in muscle repair and regeneration.
- Skin Cells: Skin stem cells help regenerate epidermal layers, making them useful in burn treatments and wound healing.
3. Induced Pluripotent Stem Cells (iPSCs)
These are genetically reprogrammed adult cells that behave like embryonic stem cells. By introducing specific genes, scientists can convert ordinary somatic cells (such as skin or blood cells) into pluripotent stem cells, eliminating ethical concerns associated with embryonic stem cells. iPSCs hold great potential for personalized medicine, organ regeneration, and drug testing.
Types of Stem Cell Therapy
Stem cell therapy has numerous applications in medicine, ranging from tissue regeneration to treating chronic diseases.
1. Hematopoietic Stem Cell Transplantation (HSCT)
- Used for: Treating leukemia, lymphoma, sickle cell anemia, and immune disorders.
- How it works: Stem cells from bone marrow or umbilical cord blood are transplanted into a patient to restore normal blood and immune function.
2. Mesenchymal Stem Cell Therapy (MSC Therapy)
- Used for: Repairing bone, cartilage, and muscle tissues.
- How it works: MSCs, obtained from bone marrow, fat, or umbilical cord, are injected into damaged areas to promote healing.
- Applications: Treatment of arthritis, osteoporosis, spinal cord injuries, and heart disease.
3. Neural Stem Cell Therapy
- Used for: Treating neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and spinal cord injuries.
- How it works: Neural stem cells differentiate into neurons and glial cells, replacing damaged brain and spinal cord tissues.
4. Cardiac Stem Cell Therapy
- Used for: Repairing heart tissue after a heart attack or heart failure.
- How it works: Stem cells stimulate the regeneration of damaged heart muscles, improving heart function.
5. Skin and Corneal Stem Cell Therapy
- Used for: Treating severe burns, corneal blindness, and skin disorders.
- How it works: Stem cells are used to regenerate skin layers or repair damaged corneal tissue, restoring vision and skin integrity.
6. Pancreatic Stem Cell Therapy
- Used for: Treating diabetes by regenerating insulin-producing beta cells in the pancreas.
- How it works: Stem cells are programmed to develop into insulin-producing cells, potentially curing Type 1 diabetes.
Applications of Stem Cell Therapy
Stem cell therapy is revolutionizing medicine with applications in multiple fields:
1. Regenerative Medicine and Tissue Engineering
- Stem cells aid in organ repair and regeneration, reducing the need for organ transplants.
- Engineered tissues can replace damaged skin, cartilage, and even entire organs.
2. Personalized Medicine
- Patient-specific iPSCs can be used for drug testing, minimizing side effects.
- Customized stem cell treatments can be tailored to an individual’s genetic makeup.
3. Cancer Treatment
- Bone marrow transplants help restore blood-forming cells after chemotherapy and radiation therapy.
- Stem cell-based immunotherapy enhances cancer-fighting immune cells.
4. Autoimmune Disease Treatment
- Stem cell therapy is being explored for treating multiple sclerosis (MS), rheumatoid arthritis, and lupus by resetting the immune system.
5. Organ Transplantation Alternatives
- Scientists are working on bioengineering organs using stem cells, which could eliminate organ shortages and transplant rejection risks.
Challenges and Ethical Considerations
Despite its promising potential, stem cell therapy faces scientific, ethical, and regulatory challenges:
1. Ethical Concerns
- Embryonic stem cell research involves the destruction of human embryos, raising moral and religious debates.
- Cloning and genetic modifications using stem cells may lead to ethical dilemmas in human enhancement.
2. Safety Issues
- The risk of tumor formation (teratomas) due to uncontrolled stem cell growth.
- Potential immune rejection in allogeneic (donor-derived) stem cell transplants.
3. Scientific Limitations
- Limited differentiation control: Scientists are still learning how to guide stem cells into specific cell types efficiently.
- High costs and accessibility: Stem cell therapies remain expensive and are not yet widely available for many conditions.
Future of Stem Cell Therapy
As stem cell research advances, future developments include:
- 3D Bioprinting of Organs: Using stem cells to print fully functional organs.
- Stem Cell Banks: Storing umbilical cord blood and iPSCs for future medical use.
- Gene Editing with CRISPR: Combining stem cell therapy with genome editing for precision medicine.
- Enhanced Drug Discovery: Using patient-derived stem cells to test new drugs in personalized treatments.
Stem cell therapy is paving the way for a new era in medicine, offering hope for incurable diseases, enhanced healing, and personalized treatments. As research progresses, stem cells could transform healthcare, leading to longer, healthier lives for millions worldwide.
BIOTECHNOLOGICAL APPLICATION IN AGRICULTURE
Genetically modified (GM) crops have emerged as one of the most transformative technologies in modern agriculture, offering enhanced qualities such as increased yield, improved nutritional profiles, and resistance to pests and diseases. By using advanced genetic engineering techniques, scientists are able to introduce, silence, or modify specific genes to achieve desired traits. These crops include well-known examples like Golden Rice and Bt cotton, which have been developed to address both agricultural and nutritional challenges.
Innovations in GM Crops
Bt Cotton and Bt Brinjal
- Bt Cotton: Bacillus thuringiensis (Bt) is a soil bacterium whose toxin genes have been incorporated into cotton plants to provide resistance against bollworms. This modification greatly reduces the need for chemical pesticides, leading to environmentally friendly farming practices and lower production costs. India pioneered GM crop technology with Bollgard I, a single-gene Bt cotton introduced in 2002, followed by Bollgard II, a double-gene technology approved for commercialization in mid-2006.
- Bt Brinjal: The insertion of the cry1Ac gene from Bacillus thuringiensis into brinjal has resulted in Bt brinjal, a transgenic variety engineered to resist pests such as the Brinjal Fruit and Shoot Borer (Leucinodes orbonalis). This genetic modification helps protect the crop from insect damage, thereby increasing yield and reducing the reliance on chemical insecticides.
Golden Rice and Nutritional Enhancement
- Golden Rice: To combat malnutrition, particularly vitamin A deficiency in developing countries, scientists have biofortified rice with vitamin A. Golden Rice is engineered to produce beta-carotene, a precursor of vitamin A, thereby enhancing its nutritional value and offering a sustainable solution to micronutrient deficiencies.
- Enhanced Nutritional Content: Beyond Golden Rice, other genetically modified crops such as biofortified wheat have been developed to have higher iron or protein contents. These innovations not only aim to improve crop yields but also enhance the overall dietary quality of staple foods.
Techniques and Approaches in GM Crop Production
GM crops are produced using several sophisticated techniques that ensure precision and efficacy:
Gene Modification and Transgenic Techniques
- Gene Silencing and Cytoplasmic Male Sterility: These methods are used to control gene expression and improve hybrid seed production. Gene silencing can effectively shut down specific genes, while cytoplasmic male sterility is useful in breeding programs to produce hybrid crops without manual emasculation.
- Selectable Markers and Cloning Sites: During the creation of recombinant DNA, scientists use restriction enzymes to cut DNA at specific sequences and mix these fragments with DNA from other organisms. Selectable markers help identify successfully modified organisms, while designated cloning sites facilitate the insertion of foreign DNA.
Techniques for Inserting DNA
- Microinjection:
In animal systems, DNA is directly injected into the nucleus of cells using microinjection techniques. - Biolistics:
In plants, DNA-coated metal particles are bombarded into cells using a gene gun, ensuring the incorporation of new genetic material. - Polymerase Chain Reaction (PCR): PCR is employed to amplify the target gene, creating multiple copies that can be introduced into the host organism.
- Cloning Vectors: Plasmids and other vectors are used to transport the recombinant DNA into host cells. Essential vector components, such as the origin of replication and promoter sequences, ensure the foreign DNA is replicated and expressed efficiently.
- Microinjection:
Benefits and Sustainable Practices
Resistance to Pests and Diseases
- Bt Crops: Crops engineered with Bt bacterial Cry proteins provide robust protection against a range of insect pests, including cotton bollworms and crop borers. This resistance reduces the dependency on chemical pesticides, leading to healthier ecosystems and lower production costs.
Herbicide Resistance
- Roundup-Ready Soybeans: Herbicide-resistant crops enable farmers to manage weeds more effectively without harming the crop itself. This selective resistance not only simplifies weed control but also minimizes the overall use of herbicides in farming.
Advanced Propagation Techniques
- Micropropagation and Tissue Culture: These laboratory techniques facilitate the rapid growth of plants, ensuring uniformity and disease-free planting material. They are essential for scaling up the production of GM crops and maintaining consistent quality across large fields.
Biopesticides and Biofertilizers
- Environmentally Friendly Alternatives: Biopesticides and biofertilizers, derived from bacterial, fungal, or plant sources, offer sustainable alternatives to chemical inputs. They naturally combat pests and fix nitrogen, promoting sustainable farming practices while reducing environmental impact.
Food Fortification and Biofortification
Food Fortification
Food fortification involves the addition of essential vitamins and minerals to commonly consumed foods during processing. This cost-effective strategy has been widely adopted to prevent micronutrient deficiencies and improve public health on a large scale.
Biofortification
Biofortification is the process of breeding food crops with enhanced nutritional profiles. By increasing levels of iron, zinc, amino acids, and provitamin A carotenoids in crops, biofortification initiatives aim to combat malnutrition and improve dietary outcomes, especially in regions where access to diverse foods is limited.
Regulations and Oversight of GM Crops in India
The development and commercialization of GM crops in India are governed by strict regulatory frameworks:
- Environmental Protection Act (EPA), 1986: The EPA laid the groundwork for regulating genetically modified organisms (GMOs) and their byproducts. Guidelines established under this act, first published in 1989, provide comprehensive instructions for the safe handling and use of GMOs.
- Genetic Engineering Appraisal Committee (GEAC): Operating under the Ministry of Environment, Forests, and Climate Change (MoEF&CC), the GEAC is responsible for evaluating the environmental impact of GM crops and other recombinant DNA research. It ensures that any widespread use of GMOs is conducted in a manner that safeguards the environment and public health.
The Future of GM Crops
Genetically modified crops continue to evolve, with ongoing research focused on increasing their efficacy, safety, and sustainability. As biotechnology advances, future GM crops are expected to:
- Enhance nutritional profiles further to address global health challenges.
- Improve resistance to biotic and abiotic stresses, ensuring crop resilience in the face of climate change.
- Support sustainable agricultural practices by reducing reliance on chemical inputs and promoting ecological balance.
With robust regulatory frameworks and continuous scientific innovation, GM crops hold the promise of transforming agriculture, enhancing food security, and contributing to a more sustainable and healthier world.
BIOTECHNOLOGICAL APPLICATION IN MEDICINE
Modern biotechnology has revolutionized the way we approach medical treatment, disease prevention, and personalized healthcare. From producing life-saving proteins to tailoring treatments based on genetic profiles, several advanced techniques have emerged to improve patient outcomes and quality of life. Below are some key biotechnological applications that are shaping the future of medicine.
Recombinant DNA Technology
Recombinant DNA technology enables scientists to produce essential proteins by combining genetic material from different sources. One of its most notable achievements is the production of recombinant insulin. By inserting the insulin gene into bacteria, researchers have created a reliable and cost-effective source of insulin, which is widely used in diabetes management. In addition to insulin, recombinant human growth hormone is produced using similar methods, significantly improving the treatment of growth disorders. This technology has opened the door to the mass production of various therapeutic proteins that are crucial in managing a range of chronic conditions.
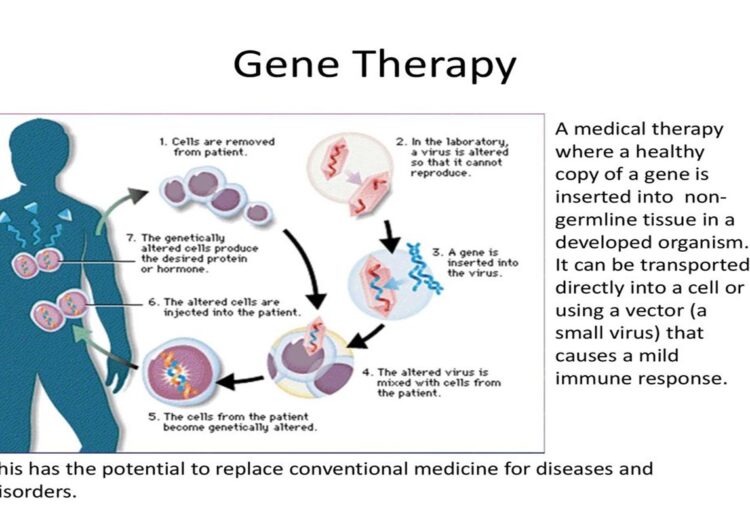
Gene Therapy
Gene therapy is a cutting-edge approach used to treat genetic disorders by correcting defective genes at the molecular level. One prominent example is the treatment of Adenosine Deaminase (ADA) Deficiency, a form of severe combined immunodeficiency (SCID). By introducing functional ADA genes into patients, gene therapy helps restore normal immune function and offers hope to those affected by inherited genetic diseases. Ongoing research in gene therapy continues to expand its applications, promising potential cures for a wide array of genetic disorders and complex diseases.
Biotechnologically Produced Vaccines
Advancements in recombinant DNA techniques have led to the development of safer and more effective vaccines. For instance, the Hepatitis B vaccine is produced biotechnologically, ensuring high purity and enhanced safety profiles compared to traditional vaccine production methods. These vaccines not only provide robust protection against diseases but also contribute significantly to public health by reducing the incidence and spread of infectious diseases.
Monoclonal Antibodies
Monoclonal antibodies are artificially produced antibodies that can target specific cells or proteins. One notable example is Rituximab, which is used in the treatment of autoimmune diseases and certain types of cancer, such as lymphoma. By specifically targeting and binding to abnormal cells or proteins, monoclonal antibodies help the immune system eliminate harmful agents with greater precision and fewer side effects than conventional therapies. This targeted approach has transformed the treatment landscape for various chronic and life-threatening conditions.
Stem Cell Therapy
Stem cell therapy, a pillar of regenerative medicine, offers promising avenues for repairing damaged tissues and organs. By using stem cells or their derivatives, clinicians can promote the regeneration of tissues affected by cardiac conditions, Parkinson’s disease, spinal cord injuries, and more. The ability of stem cells to differentiate into specialized cell types, such as heart muscle cells or nerve cells, makes them invaluable in restoring normal function and improving quality of life for patients with degenerative diseases or traumatic injuries.
Pharmacogenomics
Pharmacogenomics studies how an individual’s genetic makeup influences their response to medications. This field of research is pivotal in the development of personalized medicine, where treatments and dosages are tailored to a patient’s unique genetic profile. By understanding genetic variations that affect drug metabolism and efficacy, pharmacogenomics helps in minimizing adverse drug reactions and optimizing therapeutic outcomes. This personalized approach not only enhances patient safety but also increases the overall effectiveness of medical treatments.
Conclusion
The integration of these advanced biotechnological techniques is paving the way for a new era in healthcare. From producing vital proteins to customizing treatments based on genetic insights, these innovations are transforming the landscape of modern medicine. As research continues to evolve, these technologies hold the promise of more effective, safe, and personalized treatments that will benefit millions of patients worldwide.
BIOTECHNOLOGICAL APPLICATION IN ENVIRONMENT
Environmental biotechnology involves the application of biological processes and organisms to protect, remediate, and improve the quality of the environment. This multidisciplinary field leverages nature’s own mechanisms to tackle pollution, restore ecosystems, and promote sustainable practices in agriculture, industry, and urban development.
Innovative Technologies in Environmental Biotechnology
Biorock Technology
Biorock technology is a cutting-edge method used to rehabilitate marine ecosystems, particularly coral reefs. This innovative technique involves the use of steel structures placed on the sea floor that are connected to a power source, such as surface-floating solar panels. When electricity is applied, minerals dissolved in seawater electro-accumulate on the steel, forming a substrate called biorock. This mineral-rich material facilitates the growth of coral and other marine organisms by providing a favorable environment for settlement and regeneration. Biorock structures have been instrumental in enhancing coral resilience, accelerating reef recovery, and combating the adverse effects of climate change and ocean acidification.
Other Key Techniques
Bioremediation
Bioremediation is the process of using microorganisms to break down or neutralize harmful toxins in soil, water, or sediments. This natural detoxification process transforms pollutants into less toxic or harmless compounds, reducing risks to human health and the environment. Microbes capable of degrading industrial chemicals, petroleum products, and pesticides are often deployed to clean up contaminated sites, making bioremediation a sustainable and cost-effective alternative to conventional remediation methods.
Phytoremediation
Phytoremediation is a specific type of bioremediation that employs plants to manage environmental contaminants. It includes several mechanisms:
- Phyto-degradation: Some plants metabolize and break down pollutants within their tissues, effectively neutralizing harmful substances.
- Phyto-volatilization: Other plants absorb water containing organic contaminants and release these pollutants into the atmosphere through their leaves, transforming them into less harmful forms. This plant-based approach is particularly effective for remediating soils and groundwater, offering an eco-friendly solution for large-scale environmental restoration.
Biosensors
Biosensors are analytical devices that detect and quantify biological reactions by converting them into measurable physical, chemical, or electrical signals. These devices are vital in monitoring environmental pollutants, detecting toxins, and assessing contamination levels in various ecosystems. By providing real-time data, biosensors help researchers and environmental agencies make informed decisions regarding pollution control and ecosystem management.
DNA Barcoding
DNA barcoding involves using short, standardized DNA sequences (typically 400–800 base pairs) to identify and catalog species. By comparing the DNA barcode of an unknown sample to an extensive digital library, scientists can:
- Identify novel species and assess biodiversity.
- Evaluate food safety and trace the origin of food products.
- Detect cryptic species and monitor invasive alien species.
- Identify threatened and endangered species for conservation efforts. This method plays a crucial role in environmental monitoring, wildlife management, and ecosystem research.
Gene Silencing/Methylation
Gene silencing, primarily through DNA methylation, is the process of controlling gene expression to stop the production of specific proteins. This mechanism is used in various applications, such as:
- Agricultural biotechnology: Scientists have developed RNA molecules to inhibit fungal genes that cause groundnuts to produce aflatoxin, thereby enhancing food safety.
- Medical research: RNA interference (RNAi) is used to silence genes associated with cancer and neurodegenerative diseases, paving the way for novel treatments. Gene silencing helps elucidate gene function and regulate biological pathways, contributing to advances in both environmental and clinical biotechnology.
DNA Profiling or DNA Fingerprinting
DNA profiling, commonly known as DNA fingerprinting, is a forensic method used to identify individuals based on their unique DNA patterns. This technique relies on analyzing short, repeating DNA segments known as microsatellites. Since these polymorphic markers vary greatly among individuals, they serve as a powerful tool for:
- Criminal investigations, by matching suspects’ DNA with evidence from crime scenes.
- Medical and genetic research, by studying inheritance patterns and genetic predispositions.
- Immigration and parentage testing, ensuring accurate identification and verification. DNA profiling has become an indispensable component of forensic science and genetic analysis.
Environmental biotechnology encompasses a wide array of innovative practices and technologies that harness the power of biology to address some of the most pressing environmental challenges. From biorock technology and bioremediation to DNA barcoding and gene silencing, these methods contribute to a cleaner, healthier, and more sustainable environment. As research in this field continues to evolve, environmental biotechnology will undoubtedly play an increasingly vital role in preserving our planet’s ecosystems and ensuring the well-being of future generations
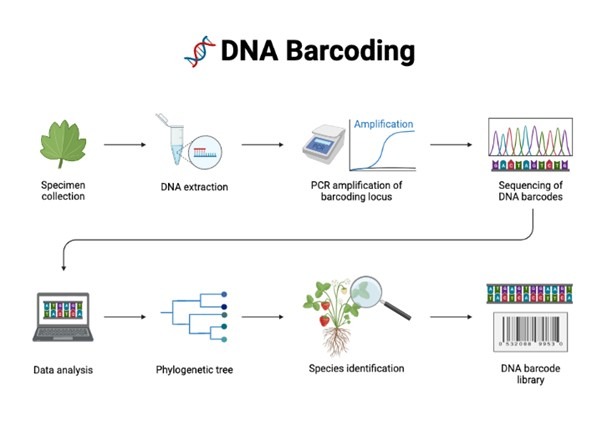
DNA BARCODING
DNA barcoding is an innovative molecular technique that utilizes a short, uniform DNA sequence—typically between 400 and 800 base pairs—to identify and differentiate between species. In theory, every species on the planet has a unique DNA barcode that can be easily created, cataloged, and described. By comparing the DNA barcode sequence from an unknown sample, whether it’s taken from a market, garden, or forest, to a vast online digital library of barcodes, scientists can quickly and accurately identify the species.
How DNA Barcoding Works
The process begins by extracting a small sample of DNA from the organism. A specific region of the genome, chosen for its consistency and variability across different species, is amplified using techniques such as the polymerase chain reaction (PCR). The resulting DNA fragment serves as the barcode—a unique genetic identifier that can be analyzed and compared against an extensive reference database. This digital library contains barcode sequences from thousands of known species, providing a comprehensive resource for identification.
Applications of DNA Barcoding
DNA barcoding has a wide range of applications that extend across multiple disciplines:
- Identifying Novel Species: DNA barcoding helps researchers discover and catalog new species, even in biodiversity-rich environments where traditional methods may fall short.
- Food Safety and Authenticity: In the food industry, DNA barcoding is used to verify the authenticity of products, ensuring that the species present in a food item match what is declared on the label. This is crucial for detecting adulteration and ensuring consumer safety.
- Detection of Cryptic Species: Some species are so similar in appearance that they are virtually indistinguishable by morphology alone. DNA barcoding enables the identification of these cryptic species by revealing genetic differences.
- Monitoring Invasive and Alien Species: Rapid identification of non-native species is essential for managing invasive species that could disrupt local ecosystems. DNA barcoding aids in early detection and monitoring, helping to protect biodiversity.
- Conservation of Endangered Species: By accurately identifying threatened and endangered species, DNA barcoding supports conservation efforts and helps monitor the health and diversity of ecosystems.
The Future of DNA Barcoding
As sequencing technologies continue to advance and databases expand, DNA barcoding will become even more powerful and accessible. Its integration into ecological research, regulatory practices, and conservation strategies promises to revolutionize our understanding of the natural world, enabling more informed decisions to safeguard biodiversity for future generations.
GENE SILENCING/METHYLATION
Gene silencing is the deliberate control of gene expression, aimed at preventing a specific gene from being expressed. This process is vital for regulating cellular functions and has become an invaluable tool in both research and therapeutic applications. One of the primary mechanisms behind gene silencing is DNA methylation—the addition of methyl groups to DNA molecules. Methylation typically occurs at cytosine bases within CpG dinucleotides, leading to a more compact chromatin structure that hinders the access of transcriptional machinery, thereby reducing or completely blocking gene expression.
For example, scientists have successfully engineered two small RNA molecules designed to inhibit the fungal genes responsible for aflatoxin production in groundnuts. Aflatoxins are potent toxins that can contaminate crops and pose severe health risks. By silencing these harmful genes, the production of aflatoxin is significantly reduced, improving food safety and crop quality.
Applications of Gene Silencing
1. Cancer Treatments
Gene silencing via RNA interference (RNAi) has emerged as a promising approach in cancer therapy. By selectively silencing oncogenes—genes that, when overexpressed or mutated, contribute to cancer development—researchers can inhibit tumor growth and proliferation. This targeted strategy not only minimizes damage to healthy cells but also opens the door for personalized medicine, where treatments are tailored to the genetic profile of an individual’s cancer.
2. Epigenomic Analysis and Clinical Molecular Diagnostics
Gene silencing is a key component in epigenomic research, where scientists investigate the complex regulatory networks that control gene expression. Understanding these networks helps in identifying biomarkers for various diseases. RNA interference-based diagnostic tools are increasingly being used to detect abnormal gene expression patterns, providing early warnings for conditions such as cancer and neurodegenerative disorders.
3. Therapy for Neurodegenerative Diseases
The application of gene silencing extends into the realm of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. By targeting and silencing genes that contribute to the progression of these disorders, researchers aim to slow or even halt disease progression. This approach holds significant promise for improving patient outcomes and quality of life.
DNA PROFILING OR DNA FINGERPRINTING
DNA profiling is a powerful forensic procedure used to identify a unique DNA pattern, or profile, in an individual or tissue sample. This technology has revolutionized criminal investigations by allowing experts to compare a suspect’s DNA profile with evidence collected from a crime scene, thereby determining their potential involvement in the crime. Beyond its forensic applications, DNA profiling is widely used in medical and genetic research, immigration eligibility assessments, and parentage testing, providing reliable and accurate identification in a variety of contexts.
How DNA Profiling Works
At the core of DNA profiling is the analysis of short, repeating DNA segments known as microsatellites. These segments, typically composed of 1 to 6 (or more) base pairs, vary greatly from person to person, making them excellent polymorphic markers. Although these sequences are non-coding and do not directly produce proteins, their high degree of variability among individuals renders them invaluable for distinguishing one genetic profile from another.
Key Components of DNA Profiling
- Microsatellite DNA (Short Tandem Repeats): These are short, repeating sequences scattered throughout the genome. The number of repeat units at each microsatellite locus varies between individuals, creating a unique genetic signature.
- Polymorphic Markers: The inherent variability in microsatellite DNA is utilized as polymorphic markers that aid forensic experts in matching DNA samples to specific individuals. This variability is also crucial for studies in inheritance patterns and population genetics.
- DNA Fingerprint Creation: By analyzing multiple microsatellite loci, scientists generate a comprehensive DNA profile or “fingerprint” that is statistically unique to each person. This profile can be compared to other samples to confirm identity or establish familial relationships.
Applications of DNA Profiling
Forensic Investigations
- Crime Scene Analysis: DNA profiles extracted from evidence such as blood, hair, or skin cells can be compared with those of suspects. This process helps in linking a suspect to a crime scene or exonerating innocent individuals.
- Cold Cases and Exoneration: Advances in DNA profiling have enabled law enforcement agencies to revisit unsolved cases and re-examine evidence, leading to the resolution of cold cases and the exoneration of wrongfully convicted individuals.
Medical and Genetic Research
- Inheritance and Genetic Studies: DNA profiling plays a critical role in understanding inheritance patterns and genetic diversity within populations. Researchers use these profiles to study the genetic basis of diseases and develop personalized medicine approaches.
- Population Genetics: By analyzing the frequency of specific microsatellite markers in different populations, scientists gain insights into human migration patterns, ancestry, and evolutionary biology.
Immigration and Parentage Testing
- Identity Verification: DNA profiling is frequently employed in immigration cases to verify familial relationships and confirm biological parentage.
- Family Reunification: In cases where documentation is lacking or disputed, DNA profiling offers a reliable method for establishing biological connections, thereby facilitating family reunification and legal processes.
DNA profiling is a cornerstone of modern forensic science and genetic research. Through the analysis of microsatellite DNA and other polymorphic markers, this technique provides unparalleled accuracy in identifying individuals and establishing genetic relationships. Its applications extend from solving crimes and resolving cold cases to advancing our understanding of human genetics and aiding in legal processes related to immigration and parentage testing. As technology continues to evolve, DNA profiling will remain an indispensable tool in both forensic laboratories and genetic research institutions worldwide.
GENETIC DISORDERS
A genetic disorder is an illness caused by a mutation or alteration in an individual’s DNA sequence. These alterations disrupt the normal function of genes, leading to various health problems that can range from mild to life-threatening. Genetic disorders are broadly classified into three primary categories, each defined by the nature of the genetic alteration involved.
Categories of Genetic Disorders
1. Single Gene Disorders
Single gene disorders are conditions caused by mutations in a single gene. These disorders often follow straightforward and predictable patterns of inheritance. When a single gene is altered, it can lead to the production of an abnormal protein or prevent the production of a vital protein altogether.
- Examples:
- Cystic Fibrosis: Caused by mutations in the CFTR gene, leading to the production of thick, sticky mucus that affects the lungs and digestive system.
- Huntington’s Disease: A neurodegenerative disorder resulting from a mutation in the HTT gene, which leads to the progressive breakdown of nerve cells in the brain.
2. Chromosomal Disorders
Chromosomal disorders occur due to variations in the number or structure of chromosomes. These abnormalities can involve missing, extra, or rearranged chromosomes, affecting multiple genes simultaneously.
Example:
- Down Syndrome: Also known as trisomy 21, this condition is caused by the presence of an extra copy of chromosome 21. It results in developmental delays, characteristic facial features, and various health issues, including heart defects.
3. Multifactorial (Complex) Disorders
Multifactorial disorders, also known as complex diseases, are caused by a combination of genetic changes in several genes and environmental factors. These conditions do not follow a simple pattern of inheritance, as lifestyle choices and environmental exposures can influence their development.
Example:
- Cancer: Often results from the accumulation of mutations in multiple genes, along with environmental factors such as cigarette smoke, dietary habits, or exposure to harmful chemicals.
Mutations: The Basis of Genetic Variation
Mutations are changes in the DNA sequence and are the primary source of variation among organisms. While some mutations have negligible effects, others can significantly impact an organism’s health or function. Over time, the accumulation of numerous mutations with minor individual effects can drive evolutionary change.
Example:
Sickle Cell Anemia Sickle cell anemia is a genetic blood disorder caused by a mutation in a single gene responsible for producing hemoglobin—the molecule in red blood cells that binds oxygen in the lungs and transports it throughout the body.
- Mechanism: In individuals with sickle cell anemia, a single point mutation alters the hemoglobin structure, resulting in red blood cells that are rigid and shaped like a “C” or sickle.
- Consequences: These abnormally shaped cells can become trapped in small blood vessels, obstructing blood flow. This blockage can lead to severe pain, infections, and organ damage over time.
Genetic disorders, whether stemming from a single gene mutation, chromosomal abnormalities, or multifactorial causes, highlight the complex relationship between our genetic makeup and our overall health. Understanding these disorders and the role of mutations not only provides insights into the mechanisms of disease but also informs the development of targeted therapies and preventive measures. Advances in genetic research continue to improve our ability to diagnose, manage, and treat these conditions, offering hope for better health outcomes in the future.