Air Pollution: Causes and Air Pollutants:
Environmental Concerns and Deterioration
Environmental deterioration refers to the gradual decline in the quality and health of the Earth’s natural systems. This degradation reduces the environment’s ability to support life, leading to profound ecological harm that affects not only wildlife and natural ecosystems but also human communities. As human activities continue to intensify, the balance of nature is increasingly disrupted, posing serious threats to sustainability and future generations.
Primary Causes of Environmental Deterioration
1. Population Growth: The rapid increase in the global population has led to heightened demand for essential resources such as water, food, land, and energy. This rising pressure often results in the over-exploitation of natural ecosystems, deforestation, and unsustainable agricultural practices.
2. Urban Development: As cities expand to accommodate growing populations, urbanization brings about significant changes in land use. Natural habitats are destroyed to make way for infrastructure, leading to habitat loss, increased waste production, and greater energy consumption, which all contribute to environmental stress.
3. Industrial Growth: Industrialization has fueled economic progress but also driven the large-scale extraction and consumption of natural resources. Many industries release pollutants into the air, water, and soil, and depend heavily on fossil fuels, contributing to greenhouse gas emissions and ecological imbalance.
Major Consequences of Environmental Deterioration
Climate Change: Excessive greenhouse gas emissions from human activities lead to global warming, rising sea levels, melting ice caps, and more frequent extreme weather events, all of which disrupt both human and natural systems.
Ocean Acidification: Increased carbon dioxide in the atmosphere dissolves into oceans, lowering pH levels and harming marine life, especially shell-forming organisms like corals and mollusks.
Decline in Biodiversity: Loss of natural habitats, pollution, and climate change contribute to the extinction of countless plant and animal species. Biodiversity loss weakens ecosystems, making them less resilient to environmental changes.
Desertification: The degradation of land in arid and semi-arid regions due to overgrazing, deforestation, and poor agricultural practices leads to desertification, reducing the land’s productivity and affecting food security.
Soil Degradation: Soil erosion, nutrient depletion, and contamination from chemicals diminish soil fertility, making it difficult to grow crops and threatening global food supplies.
Pollution: Air, water, and land pollution from industrial waste, plastics, agricultural runoff, and vehicle emissions pose serious health risks to humans and wildlife, while also damaging ecosystems worldwide.
Tackling environmental deterioration requires urgent and coordinated global action. By adopting sustainable practices, enforcing environmental regulations, and raising awareness, we can protect and restore our planet’s ecosystems for the well-being of current and future generations.
Pollution:
The introduction of detrimental physical, chemical, or biological substances (pollutants) into the environment as a result of human activities, which adversely affects humans, living organisms, and ecosystems.
Categories: Air pollution, Noise pollution, Water pollution, Soil pollution, Thermal pollution, and Radiation pollution.
Air Pollution:
Definition:
The existence of solid, liquid, or gaseous materials, including noise and radiation, in the atmosphere at levels that are harmful to health and the environment.
Causes:
- The rise in fossil fuel consumption in power generation, industrial processes, transportation, mining, and construction activities.
- The burning of coal and oil emits nitrogen and sulfur oxides, which contribute to the formation of acid rain (sulfuric and nitric acid), leading to the deterioration of structures (often referred to as Marble Cancer).
- Chlorofluorocarbons (CFCs), commonly found in aerosol products and refrigeration systems, pose a threat to the ozone layer.
- An increase in suspended particulates (such as unburned carbon and hydrocarbons) leads to the formation of smog, particularly in colder conditions, which diminishes visibility.
Major Causes of Air Pollution:
Emissions from Vehicles and Industries:
Vehicle Emissions:
- Predominant Pollutants (>80%): Carbon monoxide (CO), nitrogen oxides (NOx), and Non-Methane Volatile Organic Compounds (NMVOCs) such as benzene, ethanol, formaldehyde, cyclohexane, and acetone.
- Minor Emissions: Methane (CH₄), carbon dioxide (CO₂), sulfur oxides (SOx), and Total Suspended Particles (TSPs).
- Industrial Emissions: Significant sectors (including iron, steel, cement, sugar, paper, fertilizers, copper, and aluminum) emit Suspended Particulate Matter (SPM), SOx, NOx, and CO₂.
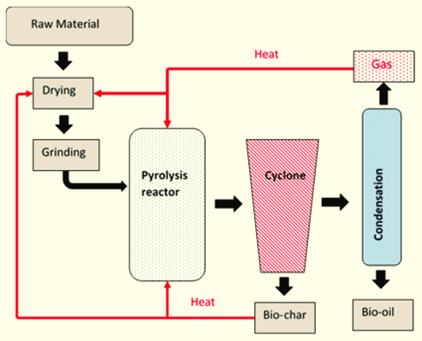
Pyrolysis: A Viable Alternative Method
Pyrolysis is an innovative thermal decomposition process that has emerged as a promising solution for sustainable waste management and energy production. It involves the chemical breakdown of synthetic and organic materials at elevated temperatures—typically between 300°C and 400°C—in the absence of oxygen. This controlled environment prevents combustion and results in the generation of valuable by-products such as fine carbon residue (char), pyrolysis gas (pyro-gas), and pyrolysis oil.
Through this process, waste materials that would otherwise occupy landfills or contribute to pollution can be transformed into usable resources, supporting both environmental and economic sustainability.
Challenges and Environmental Concerns
Despite its potential, pyrolysis is not without its drawbacks. Many tire recycling and industrial facilities utilize inefficient pyrolysis technologies, which can lead to increased carbon emissions and environmental hazards. Poorly managed systems may release toxic compounds, offsetting the benefits of the process. As such, the development and implementation of cleaner, more efficient pyrolysis methods are critical to maximizing its sustainability.
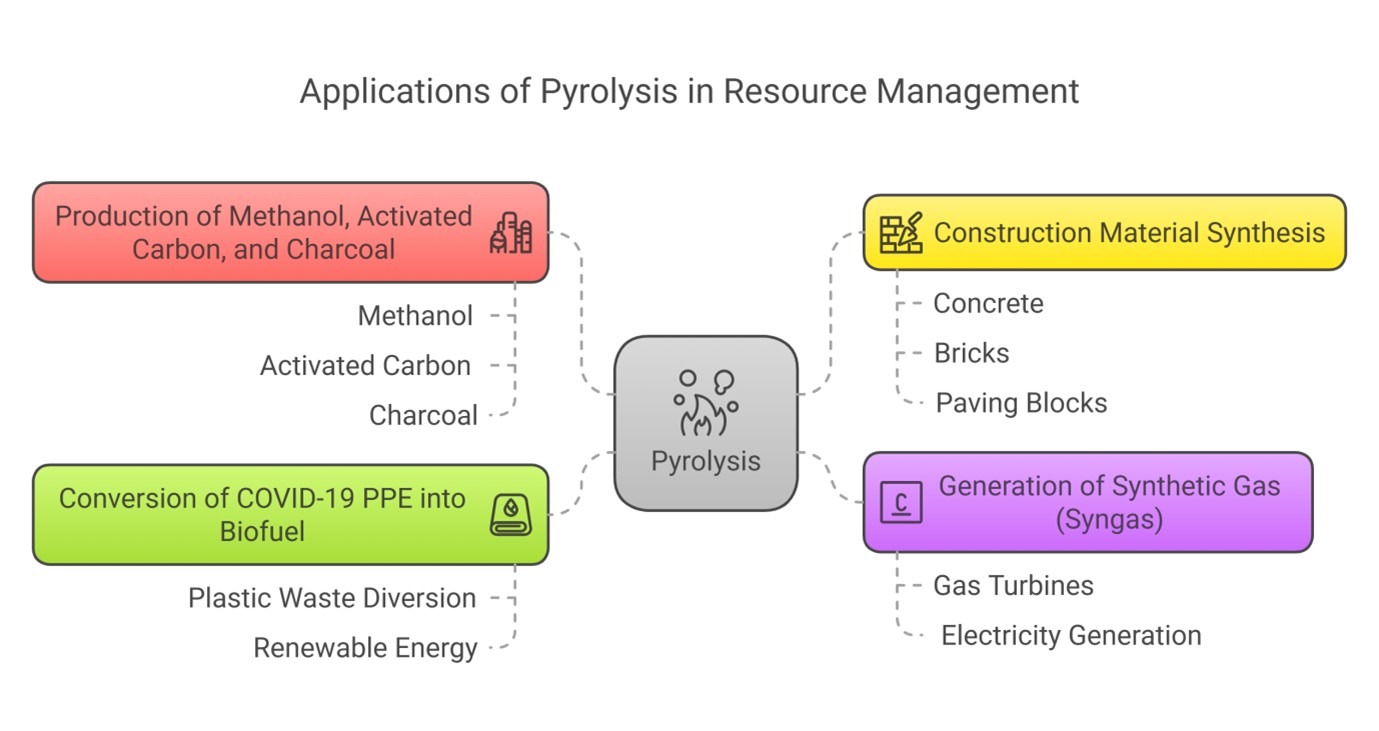
Applications of Pyrolysis
Pyrolysis has a wide range of practical applications across various industries:
1. Production of Methanol, Activated Carbon, and Charcoal– Biomass pyrolysis, especially using wood, can yield methanol and activated carbon—both valuable in industrial processes and water purification. Charcoal derived through pyrolysis serves as a clean-burning fuel and soil amendment.
2. Generation of Synthetic Gas (Syngas) – Pyrolysis gases can be converted into synthetic gas, which is used to generate electricity through gas turbines. This provides a renewable energy source and reduces reliance on fossil fuels.
3. Construction Material Synthesis– The solid by-products of pyrolysis—such as soil-like residue, ceramics, stone fragments, and glass—can be repurposed in the construction industry. These materials are incorporated into concrete, bricks, and paving blocks, promoting resource conservation and circular economy practices.
4. Conversion of COVID-19 Personal Protective Equipment (PPE) into Biofuel: An innovative and timely application of pyrolysis is the transformation of discarded PPE from the COVID-19 pandemic into biofuel. This process not only diverts harmful plastic waste from landfills and oceans but also provides a renewable energy alternative.
Benefits of Pyrolysis
Pyrolysis offers numerous environmental and economic advantages:
- Cost-Effective for Diverse Feedstocks– The process is versatile and can handle a wide range of organic and synthetic materials, including plastic, biomass, tires, and agricultural waste.
- Reduces Landfill Burden and Greenhouse Gas Emissions– By converting waste into usable products, pyrolysis helps decrease the amount of material sent to landfills. It also significantly lowers the release of methane and other harmful greenhouse gases.
- Minimizes Water Pollution Risks: Unlike some traditional waste treatment methods, pyrolysis does not produce leachate or wastewater, reducing the risk of contaminating water sources.
Regulatory Measures and Environmental Policy
Recognizing the environmental hazards of open burning, the National Green Tribunal (NGT) of India took decisive action in 2014 by banning the open burning of waste and the use of scrap tires as fuel in brick kilns. This measure aimed to curb air pollution, reduce toxic emissions, and promote the adoption of cleaner technologies like controlled pyrolysis. Regulatory efforts continue to play a pivotal role in ensuring the responsible use of pyrolysis and encouraging investments in modern, eco-friendly systems.
Causes and Effects of Air Pollution:
Fuel Adulteration:
Fuel adulteration refers to the illegal or unethical blending of cheaper fuels such as kerosene with more expensive, taxed fuels like diesel and gasoline. This practice is particularly common in regions where diesel and petrol are subject to high excise taxes, making them costly compared to subsidized kerosene, which is intended primarily for domestic use, especially for cooking in lower-income households.
While adulteration may provide short-term financial relief to some consumers or distributors, the long-term environmental and health consequences are dire. When adulterated fuels are burned, they release a higher concentration of harmful pollutants, including:
- Carbon Monoxide (CO): A colorless, odorless gas that reduces the oxygen-carrying capacity of the blood.
- Nitrogen Oxides (NOx): A group of gases that contribute to smog formation, acid rain, and respiratory problems.
- Particulate Matter (PM): Tiny particles that penetrate deep into the lungs, potentially causing cardiovascular and respiratory diseases.
- Secondary Emissions: The interaction of these pollutants with atmospheric elements can generate ground-level ozone and other hazardous compounds.
This issue underscores the need for strict regulatory oversight, enhanced fuel quality monitoring systems, and public awareness campaigns to discourage and eliminate such practices.
Agricultural Practices:
Modern agricultural activities, while essential for food production, are also significant contributors to atmospheric pollution. Several key pollutants are associated with the use of fertilizers, livestock management, and crop residue burning:
- Ammonia (NH₃): Released primarily from the application of nitrogen-based fertilizers and animal waste, ammonia reacts in the atmosphere to form secondary particulate matter, worsening air quality.
- Methane (CH₄): A potent greenhouse gas emitted during rice cultivation (due to anaerobic decomposition in paddy fields) and from livestock digestion processes.
- Nitrous Oxide (N₂O): Another powerful greenhouse gas, nitrous oxide is released from fertilized soils and manure management systems and contributes significantly to global warming.
Mitigating these emissions involves promoting sustainable farming practices such as precision fertilization, integrated pest and nutrient management, and livestock diet optimization.

Waste Management:
Improper or poorly managed waste disposal systems contribute significantly to the release of environmentally harmful gases, particularly from landfills and wastewater treatment facilities:
- Methane (CH₄): Decomposition of organic waste in landfills under anaerobic conditions produces methane, which has over 25 times the global warming potential of carbon dioxide over a 100-year period.
- Ammonia (NH₃): During composting and organic waste breakdown, ammonia is emitted and can contribute to secondary air pollution and eutrophication when deposited on land or water bodies.
To combat these issues, modern waste management solutions—such as biogas recovery, composting optimization, and methane capture technologies—must be implemented at a broader scale.
Stubble Burning:
Stubble burning is a traditional agricultural practice in which farmers set fire to the remaining crop residues—primarily straw stubble—after harvesting rice or wheat. Though cost-effective and time-saving for farmers, this practice has severe environmental implications.
- Regions Affected: The states of Punjab, Haryana, and Uttar Pradesh are the most affected by this issue, with burning typically occurring from September to November.
- Impact on the National Capital Region (NCR): During this period, prevailing weather conditions and wind patterns transport smoke and pollutants toward Delhi and the surrounding NCR, leading to a dramatic spike in air pollution levels and significantly reducing air quality.

The pollutants released include large quantities of particulate matter (PM2.5 and PM10), carbon monoxide, and volatile organic compounds (VOCs), which contribute to smog formation and public health emergencies.
Addressing this issue requires multi-stakeholder engagement involving government policy reform, incentives for farmers to adopt no-burn practices, and increased availability of crop residue management equipment like happy seeders and bio-decomposers.
Legal Framework:
- Considered illegal under the Indian Penal Code (IPC) and the Air Pollution Control Act of 1981.
- The National Green Tribunal (NGT) has prohibited residue burning in Rajasthan, Haryana, Punjab, and Uttar Pradesh, although enforcement of this ban is often inconsistent.
Consequences of Stubble Burning:
- Pollution: The process emits harmful gases such as methane (CH₄), carbon monoxide (CO), and volatile organic compounds (VOCs), contributing to smog and significant health risks.
- Degradation of Soil Fertility: This practice depletes essential soil nutrients and disrupts beneficial microbial communities.
- Reduction of Soil Moisture: The heat generated from burning penetrates the soil, leading to a decrease in moisture levels.
Household Air Pollution (Indoor Air Pollution):
- It arises from the combustion of different fuels such as coal, charcoal, wood, agricultural waste, animal dung, and kerosene, particularly in environments with inadequate ventilation.
- This process generates a variety of harmful pollutants, including fine particulate matter (PM2.5), black carbon, carbon dioxide, carbon monoxide, and methane.
- Additionally, materials found within the home, such as paints, carpets, and furniture, can release volatile organic compounds (VOCs) into the air.
Volcanism: Effects on Acid Rain and the Ozone Layer:
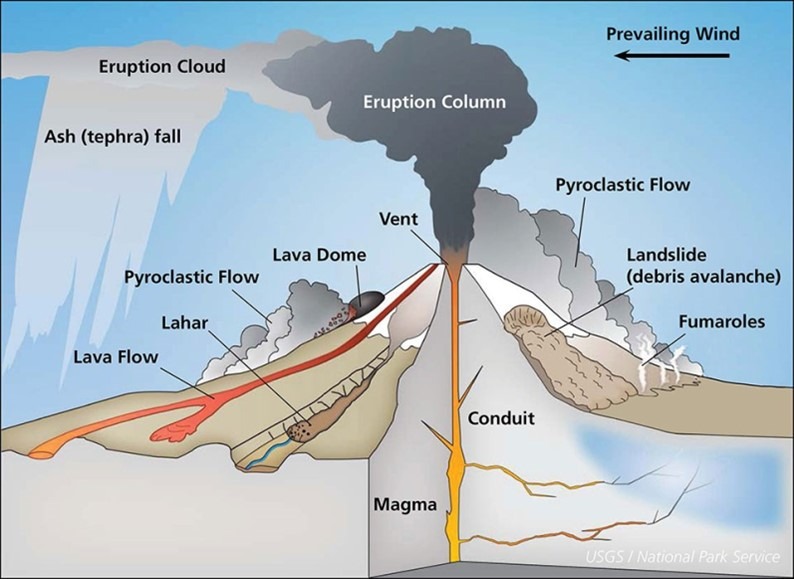
Volcanic activity is one of the most powerful natural forces on Earth, capable of shaping landscapes, influencing climate patterns, and altering atmospheric chemistry. While volcanic eruptions are often associated with dramatic visuals such as flowing lava and towering ash clouds, their environmental impacts—particularly on acid rain formation and ozone layer depletion—are equally significant, albeit less visible.
Hazardous Gases Emitted by Volcanoes
During an eruption, volcanoes emit a wide range of gases into the atmosphere. Some of these gases are highly reactive and play a major role in atmospheric changes both at the local and global levels. The most common volcanic gases include:
- Sulphur Dioxide (SO₂): A highly reactive gas that is a major contributor to acid rain and stratospheric aerosol formation.
- Carbon Dioxide (CO₂): A greenhouse gas that adds to the Earth’s atmospheric CO₂ levels, though its contribution from volcanism is relatively minor compared to anthropogenic sources.
- Hydrogen Fluoride (HF): A toxic gas that can cause harm to vegetation, livestock, and human health in areas surrounding volcanic activity.
- Hydrogen Sulphide (H₂S): Known for its characteristic “rotten egg” smell, this gas is toxic in high concentrations and contributes to air quality degradation.
Local Environmental Impacts of Volcanic Gases
Volcanic emissions, particularly sulphur dioxide (SO₂), have immediate and serious consequences for the environment in regions located downwind of an eruption site:
- Acid Rain Formation: When SO₂ reacts with water vapor in the atmosphere, it forms sulfuric acid (H₂SO₄). This acid precipitates as acid rain, which can damage forests, corrode buildings, and acidify lakes and soils, disrupting local ecosystems and agriculture.
- Air Pollution and Respiratory Health Risks: Elevated SO₂ concentrations can irritate the eyes, throat, and respiratory system, posing a health hazard to people living nearby. The fine particulate matter formed from sulphur compounds also reduces air quality and visibility.
Global Consequences of Volcanism: Sulphur Aerosols and Climate Effects
Beyond the regional effects, major volcanic eruptions can have far-reaching implications for global atmospheric dynamics, primarily through the formation of sulphur aerosols in the stratosphere:
1. Cooling of the Earth’s Surface: Volcanic sulphur dioxide, once injected into the stratosphere, reacts to form tiny sulphuric acid droplets known as aerosols. These aerosols have the ability to reflect incoming solar radiation back into space, which leads to short-term cooling of the Earth’s surface. This phenomenon has been observed after large eruptions such as Mount Pinatubo in 1991, which caused a temporary global temperature drop of about 0.5°C.
2. Degradation of the Ozone Layer: The presence of sulphur aerosols in the stratosphere also alters the chemical composition of the atmosphere, creating conditions that accelerate the breakdown of ozone (O₃) molecules. These reactions enhance the activity of chlorine and bromine compounds—substances responsible for ozone depletion. The thinning of the ozone layer allows more ultraviolet (UV) radiation to reach the Earth’s surface, increasing the risks of skin cancer, cataracts, and harming sensitive ecosystems.
Balancing Natural and Human-Induced Factors
While volcanic eruptions are natural events, their environmental effects highlight the complex interactions within the Earth’s atmosphere. Unlike human-caused emissions, volcanic pollution is sporadic and difficult to predict. However, the similarities in the chemical processes—such as acid rain formation and ozone depletion—demonstrate the need for a unified approach to managing both natural and anthropogenic atmospheric disturbances.
Volcanism, though a natural phenomenon, plays a critical role in influencing both regional and global environmental conditions. From the formation of acid rain to the alteration of climate and atmospheric chemistry, volcanic gases have the potential to impact life on Earth in significant ways. Understanding these effects is essential for enhancing global preparedness and fostering more resilient environmental policies in the face of both natural and human-driven changes.
Noise Pollution:
- Noise is defined as any undesirable sound that leads to annoyance, irritation, or discomfort for the human ear.
- It is quantified in decibels (dB), which reflect the intensity of the sound. The World Health Organization (WHO) recommends an ideal noise level of 45 dB during the day and 35 dB at night.
- The human ear can endure noise levels up to 85 dB; however, exposure to sounds exceeding this threshold can negatively impact quality of life.
- Sounds above 80 dB are classified as loud and potentially harmful, while those ranging from 100 to 125 dB are deemed uncomfortable.
Permissible Noise Levels in India:
Zone: The Central Pollution Control Board has established permissible noise levels for various zones in India. All machinery operating in these areas must adhere to the designated noise limits.
Zone | Daytime | Night time |
Industrial Zone | 75 (dB) | 70 (dB) |
Commercial Zone | 65 (dB) | 55 (dB) |
Residential Zone | 55 (dB) | 45 (dB) |
Silent Zone | 45 (dB) | 40 (dB) |
The silent zone encompasses areas within 100 meters of schools, colleges, hospitals, and courts.
Legislation to Mitigate Noise Pollution:
Initially, noise pollution and its sources were managed under the Air (Prevention and Control of Pollution) Act of 1981. Presently, they are governed separately by the Noise Pollution (Regulation and Control) Rules of 2000, which fall under the Environment (Protection) Act of 1986.
- Furthermore, noise standards for vehicles, air conditioners, refrigerators, diesel generators, and specific construction equipment are outlined in the Environment (Protection) Act of 1986.
- Noise produced by industrial activities is regulated by State Pollution Control Boards (SPCBs) in accordance with the Air (Prevention and Control of Pollution) Act of 1981.
Classification of Air Pollutants:
Types of Pollutants:
Primary Pollutants:
- These pollutants remain unchanged in their original state after being released into the environment.
- Examples include: DDT, plastics, carbon monoxide (CO), carbon dioxide (CO₂), nitrogen oxides (NOx), and sulfur oxides (SOx).
Secondary Pollutants:
- These pollutants are generated through the interactions of primary pollutants.
Examples include:
- Peroxyacetyl Nitrate (PAN): Created from the reaction between NOx and hydrocarbons.
- Ozone: Formed through:
- The combination of hydrocarbons (HC) or volatile organic compounds (VOCs) with NOx in the presence of sunlight.
- The reaction of nitrogen oxide (NO) with atmospheric oxygen.
- Acid rain, which results from the reaction of SOx and NOx with rainwater.
Quantitative Pollutants:
- These are naturally occurring substances that become pollutants when their concentrations surpass certain threshold levels.
- Examples include: Carbon dioxide (CO₂) and nitrogen oxide (NOx).
Qualitative Pollutants:
- These are synthetic pollutants that do not occur naturally in the environment.
- Examples include: Fungicides, herbicides, and DDT.
Air Pollutants:
Particulate Pollutants
- Particulate pollutants are matter suspended in the air, such as dust and soot. Their size ranges from 0.001 to 500 micrometres (µm) in diameter.
- Particles less than 10 µm float and move freely with the air current. Particles which are more than 10 µm in diameter settle down. Particles less than 0.02 µm form persistent aerosols.
- Major sources of suspended particulate matter (SPM) are industries, vehicles, power plants, construction activities, oil refineries, railway yards, marketplaces, industries, etc.
- Inhalable particulate matter PM10 and PM2.5 have been regarded as criteria pollutants because several studies have documented their adverse health effects.
- According to the Central Pollution Control Board (CPCB), particulate size 2.5 µm or less in diameter (PM2.5) is responsible for causing the most significant harm to human health.
- These fine particulates can be inhaled deep into the lungs. They can cause breathing and respiratory symp toms, irritation, inflammations, & pneumoconiosis (a disease of the lungs caused due to inhalation of dust.
- It is characterised by inflammation, coughing, and fibrosis – excess deposition of fibrous tissue).
Particulate Matter (PM2.5 and PM1):
PM2.5:
- Particles with a diameter less than 2.5 μm; these are 30 times thinner than a human hair.
- Origin: Generated from combustion activities such as petroleum refining and the burning of fossil fuels in automobiles and power generation facilities.
- Toxic Components: Includes carcinogenic substances like nickel and arsenic.
PM1:
- Particles measuring less than 1 μm in diameter; these are 70 times thinner than a human hair.
Issues of Concern:
- There is limited research and monitoring regarding health impacts.
- Approximately 40% of particulate matter, including PM0.7, is not subject to monitoring.
- There are no established international guidelines, including those from the World Health Organization (WHO).
Health Hazards:
- These particles can penetrate deeper into the respiratory tract and may even enter through the skin.
- They contain harmful toxins and metals that can lead to lung damage, genetic alterations, and cancer.
Origin:
- Primarily emitted from vehicle exhaust and industrial activities.
Fly Ash:
- By-products released during the combustion of coal in thermal power facilities.
- Environmental Consequences: Contributes to air and water pollution; leads to heavy metal contamination in aquatic ecosystems; adversely affects crop production through direct deposition on foliage.
Composition:
- High in oxides: Silica, alumina, iron, calcium, and magnesium.
- Contains hazardous heavy metals: Lead, mercury, cadmium, arsenic, cobalt, and copper.
- Primary oxides: Aluminium silicate, silicon dioxide (SiO₂), and calcium oxide (CaO).
Applications:
Construction:
- Can substitute up to 35% of cement with fly ash, resulting in reduced construction expenses.
- Produces durable and lightweight fly ash bricks.
- Serves as an excellent material for road embankments and concrete pavements.
Environmental Rehabilitation:
- Facilitates the reclamation of degraded lands and the filling of abandoned mines.
Agriculture:
- Improves the soil’s ability to retain water and boosts crop yields (note: deposition on leaves may hinder photosynthesis).
Regulatory Measures:
Ministry of Environment and Forests (MoEF) Directive:
- Encourages the use of fly ash-based materials in construction, road embankments, and landfills within a 100 km radius of thermal power plants.
- Requires mine-filling operations within a 50 km radius of power generation stations.
Nanoparticles (NPs):
- Size: Approximately 1/10⁹ of a meter, exhibiting significant heterogeneity.
- Natural Sources: Emitted from forest fires, volcanic activity, weathering processes, and dust storms.
- Anthropogenic Sources: Released into the air, water, soil, and sediments through industrial and mechanical activities, including wastewater sludge.
Key Characteristics:
- Transport: Capable of traveling thousands of kilometers and remaining airborne for several days.
- Reactivity: Possesses a high surface area-to-volume ratio, facilitating rapid reactions in the atmosphere and the formation of particles that can influence visibility and climate.
- Applications: Extensively utilized in nanotechnology across various domains, including electronics and biomedicine (for instance, in targeted drug delivery).
Environmental Effects
- Dust Cloud Formation: The aggregation of nanoparticles (NPs) leads to the creation of dust clouds that diminish sunlight penetration.
- Example: The Asian Brown Clouds, which carry soot and black carbon, settle on Himalayan glaciers, lowering their albedo and accelerating the melting process.
Impact on Hydroxyl Radicals (OH):
- Nanoparticles bind with hydroxyl radicals, decreasing their concentration in the troposphere and impairing the atmosphere’s ability to cleanse pollutants.
- Hydroxyl radicals are essential for breaking down pollutants such as carbon monoxide, methane, and volatile organic compounds (VOCs), thus preserving air quality.
- Ozone Depletion: Nanoparticles promote the generation of free radicals (e.g., Cl⁻) that contribute to ozone destruction.
Stratospheric Cooling and Ozone Loss:
- Nanoparticles interact with molecular hydrogen from hydrogen fuel cells, leading to increased water vapor in the stratosphere.
- This results in the formation of ice-crystal clouds, causing stratospheric cooling and the degradation of the ozone layer.
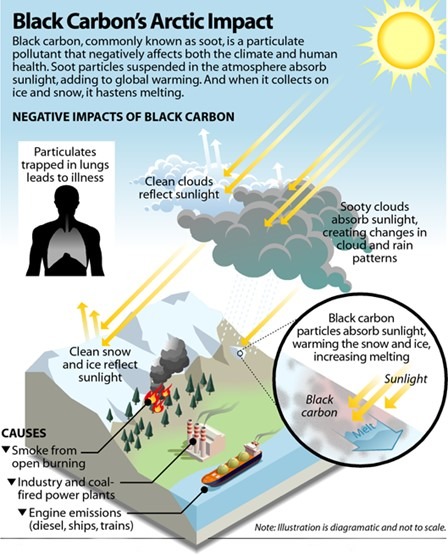
Black Carbon (Soot) and Himalayan Glaciers:
- A transient particulate pollutant, either in solid form or as an aerosol, is generated during the process of incomplete combustion.
- Regional Consequences: It interferes with cloud formation and affects monsoon precipitation patterns.
Global Consequences:
- It is the most effective absorber of sunlight, surpassing carbon dioxide in this capacity.
- It darkens snow surfaces, which decreases albedo and accelerates the melting of ice and snow.
- Persistence: This pollutant can linger in the atmosphere for several days to weeks, with its warming effects diminishing within months following reductions in emissions.
Major Global Contributors: India and China are the primary sources of these emissions.
Contribution from the Indo-Gangetic Plain:
- Approximately 20% originates from biofuels.
- Around 40% is derived from fossil fuel combustion.
- Another 40% comes from the burning of biomass.
Seasonal Fluctuations:
- The lowest levels are observed in August during the rainy season.
- The highest levels occur in May during the dry season.
Brown Carbon (BrC):
- Source: It is generated from the incomplete combustion of organic materials, such as biomass.
- Characteristics: Emitted as aerosols and brown smoke, it is distinct from black carbon, which is generally linked to combustion at lower temperatures.
Importance:
- Pollutants found in glaciers are instrumental in establishing pollution baselines and identifying their sources.
- Brown carbon significantly contributes to the acceleration of glacier melting and plays a role in climate change.
Carbon Monoxide (CO):
Key Characteristics:
- Description: A colorless, odorless, tasteless, and highly toxic gas that is slightly less dense than air.
- Longevity: It has a brief atmospheric lifespan, persisting for only a few months.
- Formation: It is produced when combustion occurs with insufficient oxygen, resulting in the formation of CO instead of CO₂. It burns with a blue flame in the presence of oxygen, transforming into CO₂.
Sources:
- Natural Origins: Generated through photochemical reactions in the troposphere, as well as from volcanic activity, forest fires, and various combustion processes.
- Anthropogenic Sources: Emitted from internal combustion engines, incomplete combustion of fuels, and as a by-product of iron smelting.
Health Effects:
- Toxicity: Becomes hazardous at concentrations exceeding 35 ppm, which can lead to carbon monoxide poisoning. It binds with hemoglobin to create carboxyhemoglobin, hindering the transport of oxygen within the body. This gas is often found in poorly ventilated areas or from overheating electronic devices, such as laptops.
Environmental Effects
- Greenhouse Gas Role: While not classified as a direct greenhouse gas, it contributes to the formation of ground-level ozone and enhances methane levels, a potent greenhouse gas.
- Ozone Formation: It plays a significant role in atmospheric processes that affect air quality.
Carbon Dioxide (CO2):
Key Characteristics
- Description: A gas that is colorless and odorless, with a density greater than that of air.
- Natural Sources: Released from volcanic activity, geothermal hot springs, geysers, and the dissolution of carbonate rocks in water or acids.
- Occurrence: It is soluble in water and can be found in various environments, including groundwater, rivers, lakes, glaciers, ice caps, and seawater.
Health Effects:
Asphyxiant Gas:
- At levels of 7%, it poses a risk of suffocation even when oxygen levels are sufficient.
- Symptoms may include dizziness, headaches, and loss of consciousness.
Environmental Effects
- Greenhouse Gas (GHG): A significant factor in global warming, primarily due to the combustion of carbon-based fuels since the onset of the industrial revolution.
- Ocean Acidification: When dissolved in water, it forms carbonic acid, which lowers ocean pH and adversely affects marine ecosystems.
Ozone (O3):
Stratospheric Ozone (Beneficial Ozone):
- This ozone is naturally produced in the stratosphere through the interaction of molecular oxygen (O₂) with ultraviolet (UV) radiation.
- It plays a crucial role in absorbing harmful UV rays, thereby safeguarding life on Earth.
Ground-Level Ozone (Harmful Ozone):
- This substance is a pollutant and a transient greenhouse gas that poses toxic risks.
- It is not emitted directly; instead, it is generated through chemical reactions involving pollutants such as carbon monoxide (CO), nitrogen dioxide (NO₂), and volatile organic compounds (VOCs) when exposed to sunlight.
- A portion of it can also be transported from the stratosphere.
Reactions Leading to Tropospheric Ozone Formation
- CO + Hydroxyl Radical (-OH) → Hydroperoxy Radical (HO₂).
- VOCs + -OH → Peroxy Radical (RO₂).
- HO₂ + Nitrogen Oxide (NO) → Nitrogen Dioxide (NO₂) + -OH/RO.
- NO₂ (through photolysis) → Ozone (O₃).
Adverse Effects of Ozone
Impact on Human Health:
- It can cause eye irritation and diminish resistance to respiratory infections such as colds and pneumonia.
- Individuals with asthma are particularly vulnerable.
Effects on Vegetation and Ecosystems:
- It harms sensitive plant life, especially during their growth periods.
- Contributes to Smog Formation: It is a key element of smog, which deteriorates air quality.
Further Considerations:
- Ground-level ozone frequently reaches hazardous concentrations on hot, sunny days.
- It can be carried over long distances by wind, affecting even remote areas.
Stratospheric Ozone Depleting Substances (ODS):
- Anthropogenic gases that emit chlorine and bromine atoms when exposed to ultraviolet radiation contribute to the depletion of stratospheric ozone, often referred to as “good ozone.”
Examples of Ozone-Depleting Substances (ODS):
- Chlorofluorocarbons (CFCs): These compounds are commonly found in aerosol products, refrigeration systems, and air conditioning units. Their use has been strictly regulated since the late 1970s due to their detrimental effects on the ozone layer.
- Hydrochlorofluorocarbons (HCFCs) and Hydrobromofluorocarbons (HBFCs): These substances were developed as alternatives to CFCs, resulting in reduced ozone depletion; however, they still function as greenhouse gases (GHGs).
- Halons: Once utilized in fire suppression systems, the production of halons was halted in developed countries in 1994.
- Methyl Bromide: This chemical is employed as a fumigant for pest management.
- Carbon Tetrachloride: Previously used in fire extinguishers, refrigeration, and cleaning products.
- Methyl Chloroform: This substance is present in aerosol formulations and serves as a solvent for cleaning metals and circuit boards.
Common Applications of ODS:
- Refrigerants utilized in air conditioning and refrigeration systems.
- Agents for foam production.
- Solvents used in industrial processes and dry cleaning.
- Propellants in aerosol containers.
- Fumigants for agricultural pest control.
Nitrogen Oxides (Oxides of Nitrogen) (NOx):
- NOx refers to a collective term for nitrogen oxides that are primarily generated during combustion processes at elevated temperatures.
Key Sources:
- Anthropogenic: Emissions from internal combustion engines and coal-fired power plants.
- Natural: Occurrences from lightning, nitrogen-fixing plants, and microbial nitrogen fixation in agricultural settings.
Formation:
- At high temperatures, oxygen and nitrogen undergo a reaction, which typically occurs in engines or boilers of power plants.
Common NOx Components:
- Nitric Oxide (NO): A gas that is both colorless and odorless.
- Nitrogen Dioxide (NO₂): A reddish-brown gas characterized by a strong odor.
- Additional components include Nitrogen Trioxide (NO₃), Nitrous Oxide (N₂O), Dinitrogen Tetroxide (N₂O₄), and Dinitrogen Pentoxide (N₂O₅).
Health and Environmental Effects:
Health Impacts:
- NOx exposure can exacerbate asthma and other respiratory conditions.
- It contributes to the formation of photochemical smog when it reacts with volatile organic compounds (VOCs) in the presence of sunlight.
Environmental Consequences:
- Acid Rain: NOx can dissolve in atmospheric moisture, leading to the production of nitric acid.
- Tropospheric Ozone: It plays a role in the formation of ozone, which adversely affects air quality.
- Global Cooling: Nitric oxide (NO) and nitrogen dioxide (NO₂) facilitate the creation of hydroxyl radicals (-OH), which help decompose methane, thereby mitigating greenhouse gas effects.
- Nitrous Oxide (N₂O): This greenhouse gas, distinct from NO and NO₂, contributes to global warming.
Sulphur Dioxide (SO2):
- A noxious gas characterized by a strong odor, it is primarily utilized in the manufacturing of sulfuric acid.
- Natural Origins: Emanates from volcanic eruptions.
Human-Made Sources:
- Combustion of coal in thermal power stations and the use of diesel fuels.
- Industrial activities, such as paper manufacturing and copper smelting.
- Chemical reactions involving hydrogen sulfide (H₂S) and oxygen.
- The roasting of sulfide minerals, including pyrite, sphalerite, and cinnabar.
Health and Environmental Consequences:
- Health Concerns: Heightens the risk of stroke, cardiovascular diseases, lung cancer, and other severe health issues that can result in early mortality.
Environmental Effects:
- Contributes to the formation of acid rain.
- Present in significant quantities in the atmosphere of Venus, attributed to volcanic activity, resulting in reflective clouds of sulfuric acid.
India’s SO₂ Emissions:
Global Context:
- In 2019, India maintained its position as the leading emitter worldwide for the fifth year in a row.
- Accounted for 21% of the global anthropogenic SO₂ emissions in 2019.
- Emission Mitigation: Achieved a 6% reduction in emissions in 2019 compared to 2018, representing the most significant decrease in four years.
- Primary Source: Coal-based electricity generation.
SO₂ Concentration Areas in India:
Key Concentration Areas (identified by OMI satellite):
- Singrauli (Madhya Pradesh), Neyveli and Chennai (Tamil Nadu), Talcher and Jharsuguda (Odisha), Korba (Chhattisgarh), Kutch (Gujarat), Ramagundam (Telangana), and Chandrapur and Koradi (Maharashtra).
- Chennai is identified as the largest urban concentration area.
Polyaromatic Hydrocarbons (PAHs):
Sources:
- These compounds are produced during the incomplete combustion of organic substances such as coal, oil, gasoline, and wood.
- They are also present in cigarette smoke and are generated when cooking meat and other foods at high temperatures.
- In the United States, naphthalene, a type of polycyclic aromatic hydrocarbon (PAH), is manufactured for use in various chemicals and as a component in mothballs.
Properties and Risks:
- Toxicity: Numerous PAHs are known to possess toxic, mutagenic, and carcinogenic properties.
- Bioaccumulation: Due to their high lipid solubility, these compounds are readily absorbed by mammals through the gastrointestinal system.
- Particulate Matter Association: PAHs are often found in conjunction with particulate matter (PM2.5 and PM10), which enhances their toxicity.
Volatile organic compounds (VOCs):
- Volatile organic compounds (VOCs) are carbon-based substances that readily evaporate at ambient temperatures.
- For instance, formaldehyde, which has a boiling point of -19 °C, is known to irritate the eyes and nose and can trigger allergic reactions.
- Common VOCs include benzene, ethylene glycol, formaldehyde, methylene chloride (commonly used as a paint remover and for metal cleaning), tetrachloroethylene (a solvent for dry cleaning), toluene, xylene, and 1,3-butadiene (utilized in the production of synthetic rubber).
- Indoor sources of these compounds are prevalent in products such as perfumes, hair sprays, furniture polish, adhesives, air fresheners, moth repellents, and wood preservatives.
- The emissions from these items can lead to various health issues, including irritation of the eyes, nose, and throat, as well as headaches, nausea, impaired coordination, and potential long-term liver damage.
- Ethylene is a significant industrial chemical primarily used in the production of polyethylene and serves as a plant hormone in agriculture to facilitate fruit ripening.
- While ethylene generally exhibits low toxicity, exposure to high concentrations can result in headaches, drowsiness, and even loss of consciousness. Its derivative, ethylene oxide, is recognized as a carcinogen.
- Formaldehyde is frequently found in materials such as particleboard, plywood, and pressed wood, as well as in fungicides, germicides, and disinfectants. It is also employed as a preservative in mortuaries and laboratories.
- Natural sources of formaldehyde include its production during the decomposition of plant matter and as a by-product of combustion processes, such as tobacco smoke.
Benzene:
- Benzene is classified as a volatile organic compound (VOC) and a polycyclic aromatic hydrocarbon (PAH). It is naturally present in crude oil, cigarette smoke, and is released during volcanic eruptions and forest fires.
- Uses: Benzene is utilized in the production of plastics, resins, synthetic fibers, and rubber lubricants, among other products.
- Fuel Properties: Its high octane rating makes benzene an essential ingredient in gasoline formulations.
Environmental and Health Effects
- As a VOC: Benzene interacts with other air pollutants, leading to the formation of ground-level ozone, which contributes to smog and can harm crops and various materials.
- Health Concerns: Prolonged exposure to benzene is associated with an increased risk of cancer and can lead to bone marrow failure.
Associated Pollutants
Toluene (Methylbenzene):
- Use: Employed as a paint thinner and as an octane enhancer in gasoline engines.
Xylene (Dimethylbenzene):
- Use: Functions as a solvent in the printing, rubber, and leather manufacturing sectors.
Styrene (Ethenylbenzene):
- Use: Serves as a primary raw material for polystyrene, which is utilized in appliances, automotive components, and more.
- A styrene gas leak at LG Polymers in Visakhapatnam in 2020 resulted in fatalities.
Minor Air Pollutants:
Lead:
- Sources: Present in petrol, diesel, lead-acid batteries, paints, and hair dyes; tetraethyl lead (TEL) serves as an anti-knock agent in petrol.
Health Effects:
- Harms the kidneys and liver, and disrupts the development of red blood cells (RBCs).
- Leads to neurological damage, gastrointestinal problems, cancer, and reduced cognitive abilities in children.
- Cumulative poisoning can occur when it contaminates water or food.
Ammonia (NH₃):
- Characteristics: A corrosive and pungent gas that is naturally released from decomposing organic matter and waste.
- Anthropogenic Sources: Arising from livestock management and the use of agricultural fertilizers.
Effects:
- Irritates the eyes, nose, and throat; contributes to the formation of ammonium salts (PM2.5) in the atmosphere.
- Promotes water nitrification and eutrophication.
Asbestos:
- A group of six fibrous silicate minerals, including chrysotile and crocidolite.
- Health Impacts: Long-term exposure can result in severe health conditions, such as lung cancer, mesothelioma, and asbestosis.
Metallic Oxides:
- Sources: Generated from dust deposition during mining and metallurgical activities.
- Impact on Plants: Induces physiological, biochemical, and developmental disorders, and can lead to reproductive failures.
Biological Pollutants:
- Examples: Include pollen, pet dander, dust mites, fungal spores, parasites, and certain bacteria.
- Health Effects: Act as allergens, triggering asthma and other allergic conditions.
Radon:
- Source: A gas that is naturally released from the soil.
- Health Risk: Can accumulate indoors due to inadequate ventilation, increasing the risk of lung cancer.
Radioactive Pollution:
Radioactivity:
The natural process by which atomic nuclei disintegrate, resulting in the emission of alpha particles (protons), beta particles (electrons), and gamma rays (high-energy electromagnetic radiation).
Types of Radiation:
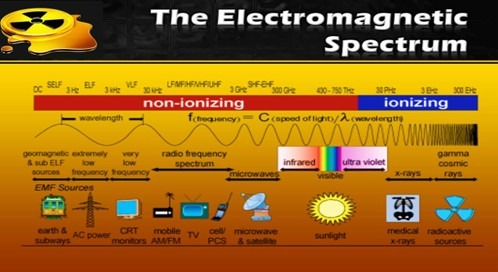
- Non-ionizing Radiation: Characterized by low energy, this type of radiation does not have the capacity to ionize atoms.
- Ionizing Radiation: This high-energy radiation can ionize atoms, potentially leading to health hazards.
Radioactive Pollution:
- The elevation of nuclear radiation levels in the environment, which poses serious risks to human health and other living organisms.
- Contamination: The unintentional presence of radioactive materials on various surfaces or within solids, liquids, or gases.
Sources of Radiation
Natural Sources:
- Cosmic rays originating from outer space.
- Terrestrial radiation emitted by radionuclides such as radium-224, uranium-238, thorium-232, potassium-40, and carbon-14.
Artificial Sources:
- Accidental releases from nuclear power facilities.
- Improper disposal of radioactive waste.
- Nuclear testing and the resultant fallout, which includes isotopes like strontium-90, caesium-137, and iodine-131.
- Extraction of radioactive materials, including uranium and thorium (e.g., from monazite ore).
- Medical procedures involving diagnostic radiation (such as X-rays and CT scans), chemotherapy, and emissions from nuclear reactors and research laboratories.
Accidents at Nuclear Power Plants:
- In the reactor core, nuclear fission produces significant heat, which, if not properly managed, can lead to the melting of fuel rods.
- Consequences: This uncontrolled process can release dangerous radioactive substances, resulting in severe repercussions for humans, wildlife, and the ecosystem.
Safety Measures and Accidents:
- Nuclear reactors are designed with various safety mechanisms to avert accidents.
- However, despite these safeguards, three major nuclear incidents have taken place:
- Three Mile Island (USA, 1979): A rise in temperature caused a core meltdown, but radiation leakage was minimal, and no immediate injuries were reported.
- Chernobyl (USSR/Ukraine, 1986): A catastrophic core meltdown resulted in a significant release of radiation, leading to numerous fatalities and extensive contamination throughout Europe.
- Fukushima Daiichi (Japan, 2011): An earthquake triggered the incident, leading to considerable environmental damage and health consequences.
Safe Disposal of Nuclear Wastes:
Types of Radioactive Waste
- Low-Level Radioactive Waste (LLW): Generated from civilian applications of radionuclides in fields such as medicine, research, and industry, as well as from materials associated with decommissioned reactors and protective gear utilized in nuclear facilities.
- High-Level Radioactive Waste (HLW): Comprises spent nuclear fuel rods and obsolete nuclear armaments.
Disposal and Management:
Current Approaches:
- Spent fuel rods are either retained in specialized storage pools at nuclear reactor sites or transported to reprocessing facilities.
- Reprocessing: Although costly, this method is employed by certain countries as an alternative to long-term waste storage.
- Example (United States): Nuclear waste is securely buried deep underground within insulated containers.
Non-Ionizing Radiation:
- Electromagnetic waves characterized by longer wavelengths include ultraviolet (UV) radiation, radio waves, and microwaves.
- Energy: These waves can excite atoms and molecules, inducing vibrations; however, they do not possess enough energy to cause ionization, meaning there is no alteration in charge.
Illustrative Examples:
- Microwave ovens utilize these waves to induce vibrations in water molecules, resulting in an increase in temperature.
- Reflection from surfaces such as sand and snow can lead to snow blindness or skin cell damage, which may result in sunburn.
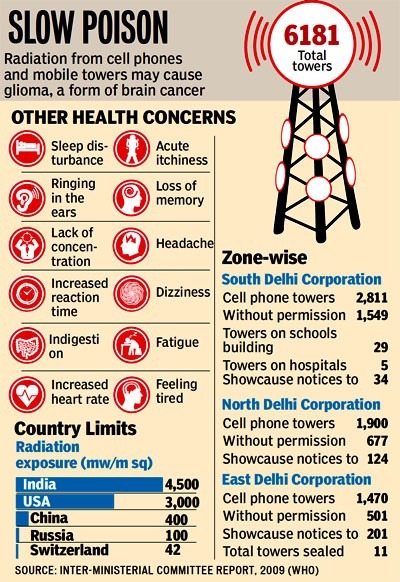
Impacts of Non-Ionizing Radiation from Cell Phone Towers:
Health Implications for Humans:
- Thermal Effects: The absorption of microwaves can lead to alterations in cellular function and psychological states, potentially resulting in genetic anomalies, developmental challenges, and effects on the nervous system.
- Non-Thermal Effects: Exposure to low-frequency radio fields may disrupt the movement of calcium and ions across cell membranes, leading to symptoms such as fatigue, headaches, irritability, and nausea.
- Current safety regulations primarily address thermal effects, often neglecting the evidence related to non-thermal exposure.
Effects on Avian Species:
- Increased Absorption: Birds, having a higher surface area-to-weight ratio, absorb radiation more readily and experience rapid heating due to their lower body fluid content.
- Navigation Disruption: The presence of magnetic fields can disorient birds, increasing the likelihood of collisions with masts and other structures.
- Ionization: Ionization is the process through which atoms or molecules either gain or lose electrons, resulting in the acquisition of positive or negative charges, frequently accompanied by chemical transformations.
Ionising Radiation:
- Ionising radiation represents one of the most potent physical forces affecting biological and environmental systems. It encompasses a range of high-energy emissions that are capable of removing tightly bound electrons from atoms and molecules, a process known as ionisation. This characteristic gives ionising radiation the ability to alter molecular structures and damage biological tissues—posing both short- and long-term risks to human health and the environment.
What is Ionising Radiation?
- Ionising radiation includes a spectrum of electromagnetic and particulate emissions, each differing in energy level, penetrability, and biological impact. These high-energy forms of radiation include:
- Short-wavelength Ultraviolet (UV) Rays
- X-rays
- Gamma rays
- Alpha particles
- Beta particles
- Neutrons (often produced during nuclear fission reactions)
- Ionising radiation includes a spectrum of electromagnetic and particulate emissions, each differing in energy level, penetrability, and biological impact. These high-energy forms of radiation include:
These emissions possess enough energy to ionise atoms by displacing electrons, leading to molecular instability and damage, especially to complex macromolecules such as DNA, proteins, and enzymes.
Mechanism of Action and Penetrability
- Ionising radiation causes direct and indirect molecular damage. In direct interactions, the radiation itself breaks chemical bonds, whereas in indirect interactions, it ionises nearby water molecules, producing reactive free radicals that damage surrounding cellular components.
- One of the defining traits of ionising radiation is its penetrative power, which surpasses that of non-ionising radiation (like visible light or microwaves). However, different types of ionising radiation vary in their ability to penetrate matter:
Penetration and Damage Potential:
- Alpha Particles (α): These are large, heavy particles with limited penetration power. They are stopped by a sheet of paper or the outer layer of human skin. However, if ingested or inhaled, alpha particles can be highly damaging to internal tissues.
- Beta Particles (β): Smaller and more energetic than alpha particles, beta particles can penetrate the skin but are typically stopped by materials such as glass, plastic, or aluminum. They pose both external and internal risks.
- Gamma Rays (γ): Gamma radiation is highly penetrative and capable of passing through human tissue, metal, and even several inches of concrete. Due to its deep tissue penetration, gamma radiation can cause widespread cellular and DNA damage.
Radioactive Half-Life: Understanding Persistence in the Environment
The half-life of a radioactive substance refers to the time it takes for half of its radioactive atoms to decay into a more stable form. This parameter is crucial for assessing environmental and health risks:
- Short half-life radionuclides decay quickly and are typically more intense over a shorter duration.
- Long half-life radionuclides persist in the environment for centuries, leading to chronic exposure risks and long-term ecological contamination.
For example, Plutonium-239 has a half-life of over 24,000 years, making it a persistent threat wherever nuclear waste is stored or released.
Radiation Dose and Health Impacts
The biological effect of ionising radiation is measured in rem (Radiation Equivalent in Man) or sieverts (Sv) in the SI system. The extent of harm depends on the type of radiation, dose received, exposure duration, and sensitivity of the affected tissues.
Dose-Dependent Effects:
- Low Doses (<1 rem): The human body can typically manage and repair minor cellular damage, with little to no observable health effects.
- Moderate to High Doses (1–100 rem): Exposure at this level can overwhelm cellular repair mechanisms, leading to radiation sickness, organ dysfunction, or increased cancer risk.
- Extremely High Doses (>100 rem): May result in severe radiation burns, permanent tissue damage, systemic failure, or even death depending on duration and exposure area.
Short-Term vs. Long-Term Effects of Exposure
Short-Term (Acute) Effects:
- Skin burns and ulcers
- Tissue necrosis
- Disruption of metabolic processes
- Nausea, vomiting, and acute fatigue
- Fatality at very high exposure levels
Long-Term (Chronic) Effects:
- Increased risk of various cancers, including leukemia and thyroid cancer
- Genetic mutations that may affect future generations
- Immune system suppression
- Shortened lifespan and premature aging
- Developmental defects in embryos and fetuses exposed in utero
Health Risks at Various Exposure Levels
- High Doses: Can cause immediate and fatal outcomes such as acute radiation syndrome (ARS), damage to the central nervous system, or multi-organ failure.
- Low to Moderate Doses (chronic exposure): Associated with increased risk of:
- Childhood leukemia
- Miscarriages and congenital abnormalities
- Low birth weight
- Cataracts
- Hair loss
- Lung fibrosis
- Decreased white blood cell (WBC) counts, increasing vulnerability to infections
Cellular and Genetic Damage
DNA and Molecular Disruption:
Gamma rays and other ionising particles can ionise water molecules surrounding DNA, generating hydroxyl radicals that react with the DNA structure, breaking chemical bonds and inducing mutations. This may result in:
- Cell cycle arrest
- DNA strand breaks
- Mutagenesis and carcinogenesis
Types of Biological Damage:
- Somatic Damage: Affects non-reproductive body cells. Consequences may include cataracts, tissue scarring, reduced immune function, and organ failure.
- Genetic (Heritable) Damage: Occurs in germ cells (sperm and egg), leading to inheritable genetic mutations. These may manifest as birth defects, developmental delays, or inherited diseases in future generations.
Ionising radiation, due to its immense energy and penetrative power, presents complex challenges for both environmental safety and public health. From nuclear power generation to medical imaging and industrial applications, understanding the mechanisms, risks, and regulations surrounding radiation is essential.
Ensuring protective measures, promoting radiation safety awareness, and advancing technologies for radiation shielding and waste disposal are critical steps toward minimizing exposure and safeguarding future generations.