Functions of an Ecosystem – Biogeo Chemical Cycling
Functions of an Ecosystem – Biogeo Chemical Cycling………………..
Types of Nutrient Cycles
Carbon Cycle (Gaseous Cycle)
Nitrogen Cycle (Gaseous Cycle)
Methane Cycle (Gaseous Cycle)
Phosphorus Cycle (Sedimentary cycle)
Sulphur Cycle (Mostly Sedimentary Cycle)
Functions of an Ecosystem – Bio geo Chemical Cycling:
Ecosystems play a fundamental role in maintaining life on Earth by facilitating two critical processes: the flow of energy and the circulation of nutrients. Energy enters an ecosystem primarily through sunlight, which is harnessed by plants during photosynthesis. This energy then moves through the food chain as organisms consume plants and other animals. However, unlike nutrients, energy cannot be recycled indefinitely; instead, it dissipates as heat during metabolic processes, eventually leaving the system permanently.
In contrast, nutrients found in organic matter follow a continuous recycling process within ecosystems. Unlike energy, they do not get used up or lost but are instead cycled through different forms indefinitely. The essential elements that make up living organisms—such as carbon, hydrogen, oxygen, nitrogen, and phosphorus—constitute about 97% of the human body’s mass and more than 95% of the total mass of all living organisms on Earth. Additionally, around 15 to 25 other elements are crucial in various forms for the survival, growth, and overall well-being of both plants and animals.
Biogeochemical Cycles: The Pathways of Nutrient Flow
The continuous circulation of essential elements between the biotic (living) and abiotic (non-living) components of an ecosystem is known as biogeochemical cycling. The term “bio” refers to living organisms, while “geo” relates to the Earth’s physical components, such as air, water, and soil. These cycles ensure that nutrients are constantly transformed and reused, allowing ecosystems to sustain life over time.
One of the most vital nutrient cycles is the carbon cycle, which regulates the movement of carbon between the atmosphere, living organisms, and the Earth’s surface. The nitrogen cycle is another crucial process that facilitates the conversion of nitrogen into various forms usable by plants and animals. Alongside these major cycles, numerous other nutrient cycles, such as those of phosphorus, sulfur, and trace minerals like iron and zinc, play indispensable roles in maintaining the delicate balance of ecological systems.
By understanding the intricate workings of nutrient cycles, we can better appreciate the interconnectedness of life and the importance of conserving natural ecosystems to ensure the continued availability of essential resources.
.
Types of Nutrient Cycles:
Nutrient cycles play a fundamental role in maintaining ecosystem balance by ensuring that essential elements continuously move between living organisms and their surrounding environment. However, not all nutrient cycles function with the same efficiency. The classification of a nutrient cycle as either perfect or imperfect depends on how quickly nutrients are replenished relative to their rate of consumption.
A perfect nutrient cycle occurs when nutrients are replaced at the same rate they are utilized, ensuring a continuous and balanced flow. In such cycles, essential elements do not accumulate in inaccessible forms or locations, making them readily available for reuse within the ecosystem.
Conversely, an imperfect nutrient cycle is characterized by a delay or inefficiency in nutrient replenishment. This imperfection arises when certain nutrients are lost from the active cycle, often becoming trapped in sediments or other non-bioavailable forms. As a result, these nutrients are rendered temporarily or permanently unavailable for immediate recycling, disrupting the ecosystem’s equilibrium.
Types of Nutrient Cycles: Gaseous vs. Sedimentary
The type of reservoir where nutrients are stored determines whether a cycle is classified as a gaseous cycle or a sedimentary cycle. Each type has distinct characteristics and plays a crucial role in sustaining life.
1) Gaseous Cycles: The Ideal Nutrient Cycles
Gaseous cycles are generally considered more efficient and “perfect” because their primary reservoirs—the atmosphere and hydrosphere—allow for relatively rapid recycling of nutrients. Since these reservoirs are dynamic and interconnected, nutrients in gaseous cycles are more easily accessible and can be quickly returned to ecosystems. Key gaseous cycles include:
- The Water Cycle (Hydrological Cycle): The continuous movement of water through evaporation, condensation, precipitation, and runoff.
- The Carbon Cycle: The exchange of carbon between the atmosphere, living organisms, oceans, and geological formations.
- The Nitrogen Cycle: The transformation of nitrogen into various forms, making it available for plant and animal use.
- The Methane Cycle: The production and breakdown of methane, a greenhouse gas with significant environmental impacts.
These cycles play an essential role in regulating atmospheric composition, maintaining climate stability, and supporting life across all ecosystems.
2) Sedimentary Cycles: The Imperfect Nutrient Cycles
Unlike gaseous cycles, sedimentary cycles involve nutrients that are stored in the Earth’s crust, primarily in rocks, minerals, and ocean sediments. Since these nutrients are not as easily exchanged between living organisms and the environment, sedimentary cycles tend to be relatively slow and imperfect. Some key sedimentary cycles include:
- The Phosphorus Cycle: Essential for DNA, RNA, and ATP production, phosphorus is primarily found in rocks and is gradually released through weathering.
- The Sulfur Cycle: Sulfur, crucial for proteins and enzymes, is cycled through the environment via volcanic activity, decomposition, and industrial processes.
- The Calcium Cycle: Calcium is vital for skeletal structures in animals and is stored in rocks before being released through natural processes.
- The Magnesium Cycle: This mineral is important for plant photosynthesis and enzyme activation, with its main reservoirs found in rocks and seawater.
Due to the slower nature of sedimentary cycles, nutrients may become locked in geological formations for extended periods, limiting their immediate availability to living organisms. This is why sedimentary cycles are considered less efficient compared to gaseous cycles.
The Importance of Nutrient Cycles in Ecosystem Sustainability
Both gaseous and sedimentary cycles are essential for the stability and sustainability of ecosystems. While gaseous cycles ensure a steady and rapid flow of nutrients, sedimentary cycles contribute to long-term nutrient storage and gradual replenishment. Understanding how these cycles function helps us appreciate the intricate connections between Earth’s biological, chemical, and geological systems.
Human activities, such as deforestation, industrial emissions, mining, and excessive fertilizer use, have significantly altered natural nutrient cycles, leading to environmental imbalances like climate change, ocean acidification, and eutrophication. By promoting sustainable practices and minimizing ecological disruption, we can help maintain the delicate balance of these vital nutrient cycles, ensuring a healthy and thriving planet for future generations
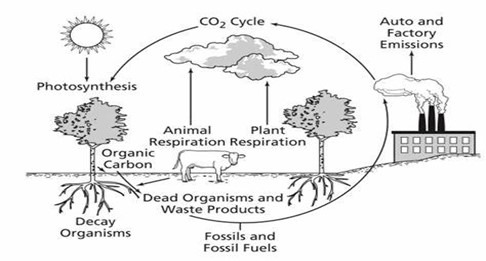
Carbon Cycle (Gaseous Cycle):
Although carbon constitutes only a small fraction of the Earth’s atmosphere compared to oxygen and nitrogen, it is an essential element for sustaining life. Carbon is the building block of all organic compounds and plays a crucial role in biological, chemical, and geological processes. It is a fundamental component of DNA, the carrier of genetic information, as well as essential organic materials such as carbohydrates, proteins, and lipids. Furthermore, carbon is stored in Earth’s geological formations as fossil fuels—coal, oil, and natural gas—created from ancient organic matter over millions of years.
One of the most important processes involving carbon is photosynthesis, in which plants and phytoplankton absorb carbon dioxide (CO₂) from the atmosphere to synthesize carbohydrates. These carbohydrates serve as the primary source of energy for the food chain, sustaining life from the smallest microorganisms to the largest animals. The movement of carbon through various reservoirs—living organisms, air, water, and geological formations—is known as the carbon cycle.
Steps in the Carbon Cycle
The carbon cycle is a continuous process that regulates the exchange of carbon between the atmosphere, living organisms, and Earth’s physical systems. The key steps in this process include:
1. Carbon Absorption via Photosynthesis
- Green plants, algae, and phytoplankton absorb carbon dioxide (CO₂) from the atmosphere through photosynthesis.
- Using sunlight, these organisms convert CO₂ and water into glucose (a form of stored energy) and release oxygen as a byproduct.
- The carbon absorbed by plants enters the food chain when herbivores consume plant matter.
2. Carbon Transfer Through the Food Chain
- Carbon moves up the food chain as animals eat plants and other animals.
- Within these organisms, carbon is utilized for growth, energy, and biological processes.
3. Carbon Release Through Respiration and Decomposition
- All living organisms, including plants, animals, and microbes, release carbon back into the atmosphere through cellular respiration.
- When organisms breathe, they convert stored glucose into energy, producing carbon dioxide as a waste product.
- When plants and animals die, decomposers (bacteria and fungi) break down their organic matter, releasing CO₂ back into the air.
4. Long-Term Carbon Storage
While most carbon cycles quickly through respiration and decomposition, some enters long-term storage in the following ways:
- Organic material can become trapped in wetlands, peat bogs, and soil sediments, where decomposition is slow due to low oxygen levels.
- In aquatic environments, carbon can combine with calcium to form insoluble carbonates, which accumulate in marine sediments over time.
- These carbon deposits eventually transform into limestone and other sedimentary rocks, locking carbon away for thousands to millions of years.
5. Geological Processes and Carbon Release
- Over long periods, tectonic activity and erosion expose buried carbon-containing rocks to surface conditions.
- When exposed, these rocks undergo weathering and chemical breakdown, releasing carbon dioxide, carbonates, and bicarbonates into rivers, lakes, and oceans.
6. Fossil Fuels and Human Impact on the Carbon Cycle
- Fossil fuels such as coal, oil, and natural gas are formed from ancient organic matter that was buried before it could decompose completely.
- Over millions of years, heat and pressure transformed this material into energy-rich hydrocarbons.
- When humans extract and burn fossil fuels, the stored carbon is rapidly released back into the atmosphere as CO₂.
- This human activity significantly disrupts the natural carbon cycle, contributing to increased atmospheric carbon levels and climate change.
The Importance of the Carbon Cycle in Ecosystem Balance
The carbon cycle is vital for regulating Earth’s climate and maintaining ecological balance. It ensures a continuous supply of carbon for living organisms while preventing excessive accumulation in any one component of the system. However, human activities—such as deforestation, burning fossil fuels, and industrial processes—are increasing carbon emissions at an unprecedented rate. This imbalance leads to global warming, ocean acidification, and disruptions in natural habitats.
By understanding the carbon cycle and promoting sustainable practices, we can help restore equilibrium and reduce the harmful effects of excessive carbon emissions. Protecting forests, adopting clean energy sources, and reducing carbon footprints are crucial steps in maintaining a stable and healthy environment for future generations.
Nitrogen Cycle (Gaseous Cycle):
The Importance of Nitrogen in Living Organisms
Nitrogen is the fourth most abundant element in living organisms and plays a crucial role in various biological processes. It is an essential component of amino acids, proteins, hormones, chlorophyll, and vitamins, making it indispensable for the growth and survival of all living organisms.
Despite its abundance in the atmosphere, where it constitutes about 78% of the air in the form of diatomic nitrogen (N₂), most organisms cannot directly use nitrogen in its elemental form. This is because atmospheric nitrogen molecules are held together by a strong triple covalent bond (N≡N), making them chemically inert. For nitrogen to be biologically useful, it must first be converted into reactive compounds like ammonia (NH₃), nitrites (NO₂⁻), or nitrates (NO₃⁻). This transformation occurs through a series of interconnected biological, chemical, and physical processes known as the nitrogen cycle.
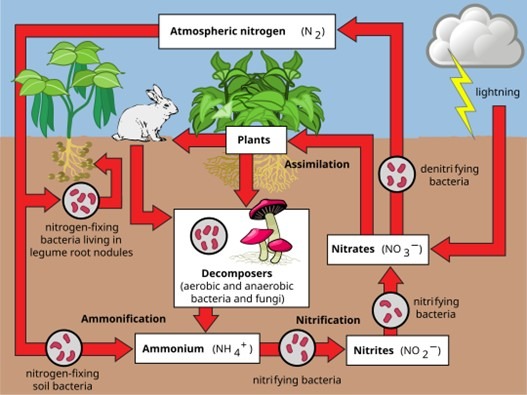
The Nitrogen Cycle: Key Processes
The nitrogen cycle describes the continuous movement of nitrogen between the atmosphere, living organisms, soil, and water. This cycle ensures that nitrogen is fixed, transformed, utilized, and returned to its natural reservoirs, maintaining ecological balance. The key stages of the nitrogen cycle include:
1. Nitrogen Fixation – Converting N₂ to Ammonia (NH₃)
Although nitrogen (N₂) is abundant in the atmosphere, most organisms cannot utilize it directly. It must first be “fixed” or converted into biologically available forms. Nitrogen fixation occurs through three primary methods:
A) Biological Nitrogen Fixation (Carried out by Microorganisms)
- Nitrogenase Enzyme Activity: Certain prokaryotic organisms, including bacteria and cyanobacteria (blue-green algae), possess a unique enzyme called nitrogenase, which can break the strong N≡N bond in atmospheric nitrogen and convert it into ammonia (NH₃) and ammonium ions (NH₄⁺).
- Free-Living Nitrogen-Fixing Bacteria: Some bacteria, such as Azotobacter (aerobic) and Clostridium (anaerobic), fix nitrogen independently in soil.
- Symbiotic Nitrogen-Fixing Bacteria: Bacteria like Rhizobium form mutualistic relationships with leguminous plants (e.g., peas, beans, and clover) by colonizing their root nodules and providing them with nitrogen.
- Cyanobacteria in Aquatic Ecosystems: Cyanobacteria such as Nostoc, Anabaena, and Spirulina play a crucial role in nitrogen fixation in marine and freshwater ecosystems.
B) Atmospheric Nitrogen Fixation (Physical Processes)
Natural phenomena such as lightning strikes and ultraviolet radiation break nitrogen molecules in the atmosphere, allowing them to combine with oxygen and form nitrogen oxides (NO, NO₂, N₂O). These oxides dissolve in rainwater and enter the soil as nitrates (NO₃⁻), which plants can absorb.
C) Industrial Nitrogen Fixation (Human Activities)
The Haber-Bosch process, used in fertilizer production, artificially combines atmospheric nitrogen (N₂) with hydrogen (H₂) to produce ammonia (NH₃), which is later used to manufacture nitrogen-based fertilizers.
2. Nitrification – Converting Ammonia to Nitrites and Nitrates
After nitrogen fixation, some plants can directly absorb ammonium ions (NH₄⁺), but most require it to be converted into nitrites (NO₂⁻) and nitrates (NO₃⁻), which are more readily absorbed by plant roots. This process, known as nitrification, occurs in two stages and is carried out by chemoautotrophic bacteria:
- Step 1: Oxidation of Ammonia to Nitrite (NO₂⁻)
- Bacteria such as Nitrosomonas and Nitrococcus oxidize ammonium ions (NH₄⁺) into nitrite (NO₂⁻).
- Step 2: Oxidation of Nitrite to Nitrate (NO₃⁻)
- The nitrite is further oxidized into nitrate (NO₃⁻) by bacteria like Nitrobacter.
- Step 1: Oxidation of Ammonia to Nitrite (NO₂⁻)
These nitrates are essential for plant growth as they serve as a key nitrogen source for synthesizing proteins, enzymes, and nucleic acids. Once absorbed by plants, the nitrogen is passed through the food chain when herbivores consume plants and carnivores consume herbivores.
3. Ammonification – Recycling Nitrogen from Organic Matter
When plants and animals die, their bodies decompose, releasing nitrogen back into the soil. Additionally, animals excrete nitrogenous wastes such as urea, uric acid, and ammonia. The process of ammonification involves:
- Decomposers such as bacteria and fungi breaking down organic nitrogenous waste into ammonia (NH₃) and ammonium ions (NH₄⁺).
- Some of this ammonia is directly used by plants, while the rest undergoes nitrification, restarting the nitrogen cycle.
4. Denitrification – Returning Nitrogen to the Atmosphere
Denitrification is the final stage of the nitrogen cycle, in which excess nitrates (NO₃⁻) in the soil are converted back into gaseous nitrogen (N₂) and released into the atmosphere. This process is carried out by denitrifying bacteria, such as:
- Pseudomonas and Clostridium, which reduce nitrates to nitrogen gas (N₂) or nitrous oxide (N₂O).
- Denitrification is particularly important in wetlands, waterlogged soils, and marine environments, where oxygen levels are low.
This process prevents the excessive buildup of nitrates in the soil and completes the nitrogen cycle by returning nitrogen to the atmosphere.
The Importance of the Nitrogen Cycle
The nitrogen cycle is essential for sustaining life, as it ensures that nitrogen remains available for plants, animals, and microorganisms. It plays a crucial role in:
1. Maintaining Soil Fertility: Nitrogen-fixing and nitrifying bacteria enrich soil with bioavailable nitrogen, promoting plant growth.
2. Supporting Food Chains: Plants use nitrogen to synthesize proteins, which are then transferred through trophic levels as animals consume plants and other animals.
3. Regulating Ecosystem Health: Excess nitrogen in the form of fertilizers can cause eutrophication (nutrient pollution), leading to algal blooms and oxygen depletion in water bodies.
4. Reducing Environmental Pollution: Denitrification helps balance nitrogen levels, preventing excessive accumulation in soil and water.
5. Enhancing Agricultural Productivity: The nitrogen cycle ensures a steady supply of nitrogen for crops, essential for global food production.
Human Impact on the Nitrogen Cycle
Human activities have significantly altered the nitrogen cycle, leading to environmental concerns such as:
- Excessive Fertilizer Use: Overuse of nitrogen-based fertilizers causes nitrate leaching, leading to water pollution and ecosystem imbalances.
- Deforestation: Reduces nitrogen-fixing plant populations, decreasing soil fertility.
- Fossil Fuel Combustion: Produces nitrogen oxides (NOₓ), contributing to air pollution, acid rain, and climate change.
- Livestock Farming: Releases large amounts of ammonia, affecting air and soil quality.
The nitrogen cycle is one of the most essential biogeochemical cycles, ensuring the continuous circulation of nitrogen through the atmosphere, biosphere, hydrosphere, and geosphere. Nitrogen is the fourth most abundant element in living organisms and is a fundamental component of amino acids, proteins, DNA, RNA, chlorophyll, and essential vitamins.
Although nitrogen (N₂) makes up approximately 78% of Earth’s atmosphere, most organisms cannot utilize it in its elemental gaseous form. Instead, nitrogen must be converted into biologically usable compounds such as ammonia (NH₃), ammonium ions (NH₄⁺), nitrites (NO₂⁻), and nitrates (NO₃⁻) before it can be absorbed by plants and subsequently passed through the food chain.
The nitrogen cycle consists of several interconnected processes that regulate nitrogen availability in the ecosystem. These processes include nitrogen fixation, nitrification, ammonification, and denitrification, each playing a crucial role in maintaining ecosystem balance.
Step-by-Step Breakdown of the Nitrogen Cycle
Step 1: Nitrogen Fixation – Converting Atmospheric Nitrogen into Usable Forms
Nitrogen gas (N₂) → Ammonia (NH₃) / Ammonium ions (NH₄⁺)
Nitrogen fixation is the process by which atmospheric nitrogen (N₂) is converted into ammonia (NH₃) or ammonium ions (NH₄⁺), making it available to plants. This conversion occurs through three primary mechanisms:
1. Biological Nitrogen Fixation (BNF):
- Certain nitrogen-fixing bacteria and cyanobacteria (blue-green algae) convert atmospheric nitrogen into ammonia using the enzyme nitrogenase.
- Examples of free-living nitrogen-fixing bacteria include:
- Aerobic bacteria: Azotobacter, Beijerinckia
- Anaerobic bacteria: Clostridium, Rhodospirillum
- Symbiotic nitrogen-fixing bacteria, such as Rhizobium, form mutualistic relationships with leguminous plants (peas, beans, clover) and some non-leguminous plants (Frankia in alder trees).
- Cyanobacteria (Nostoc, Anabaena, Spirulina) contribute to nitrogen fixation in marine and freshwater ecosystems.
2. Physical Nitrogen Fixation:
- Natural processes such as lightning and ultraviolet radiation break the strong triple bond of atmospheric nitrogen molecules, converting them into nitrogen oxides (NO, NO₂, N₂O) that dissolve in rainwater and enter the soil as nitrate ions (NO₃⁻).
3. Industrial Nitrogen Fixation:
- The Haber-Bosch process, a human-engineered method, synthetically converts atmospheric nitrogen into ammonia for fertilizer production. This has significantly boosted agricultural productivity but also contributed to nitrogen pollution.
Step 2: Nitrification – Oxidation of Ammonia to Nitrite and Nitrate
Ammonia (NH₃) / Ammonium ions (NH₄⁺) → Nitrite (NO₂⁻) → Nitrate (NO₃⁻)
- Nitrification is the biological oxidation of ammonia and ammonium ions into nitrites and then into nitrates, which can be readily absorbed by plants.
- This process is driven by specialized nitrifying bacteria:
- Step 1: Nitrosomonas and Nitrococcus oxidize ammonium ions (NH₄⁺) into nitrites (NO₂⁻).
- Step 2: Nitrobacter oxidizes nitrites (NO₂⁻) into nitrates (NO₃⁻).
- Plants primarily absorb nitrogen in the nitrate (NO₃⁻) form, which they use to synthesize essential biomolecules like proteins and nucleic acids.
Importance of Nitrification
- Converts ammonia in soil into soluble nitrates, making nitrogen more available to plants.
- However, nitrates are highly water-soluble, increasing the risk of leaching into groundwater and aquatic systems, leading to environmental issues like eutrophication.
- This process is critical in wastewater treatment systems, where nitrification is followed by denitrification to remove excess nitrogen from wastewater.
Step 3: Ammonification – Decomposition of Organic Matter
Organic matter → Ammonia (NH₃) / Ammonium ions (NH₄⁺)
Ammonification is the process by which organic nitrogen from dead plants, animals, and waste products (urea, uric acid, proteins) is decomposed by bacteria and fungi into ammonia (NH₃) and ammonium ions (NH₄⁺).
- Key decomposing microorganisms: Bacillus, Proteus, Pseudomonas, Streptomyces
- A portion of the ammonia is directly used by plants.
- Some ammonia volatilizes into the atmosphere, while the rest undergoes nitrification (Step 2) to be converted into nitrates.
Step 4: Denitrification – Conversion of Nitrate into Nitrogen Gas
Nitrate (NO₃⁻) → Nitrogen Gas (N₂) / Nitrous Oxide (N₂O)
Denitrification is the final step in the nitrogen cycle, where denitrifying bacteria convert nitrates (NO₃⁻) back into gaseous nitrogen (N₂) or nitrous oxide (N₂O), returning it to the atmosphere.
- This process occurs in anaerobic (low-oxygen) environments such as waterlogged soils, wetlands, and deep ocean sediments.
- Denitrifying bacteria include Pseudomonas, Clostridium, and Paracoccus.
- Denitrification helps regulate nitrogen levels in ecosystems and prevents excessive nitrogen accumulation in the soil and water bodies.
Summary of the Nitrogen Cycle
The nitrogen cycle follows a continuous loop that maintains nitrogen availability in different forms:
1. Nitrogen Fixation – Atmospheric nitrogen (N₂) is converted into ammonia (NH₃) or ammonium ions (NH₄⁺).
2. Nitrification – Ammonium ions (NH₄⁺) are converted into nitrites (NO₂⁻) and then into nitrates (NO₃⁻).
3. Ammonification – Organic matter decomposes, releasing ammonia (NH₃) and ammonium ions (NH₄⁺).
4. Denitrification – Nitrates (NO₃⁻) are reduced back to atmospheric nitrogen (N₂).
Complete Cycle:
Nitrogen (N₂) → (Nitrogen Fixation) → Ammonia (NH₃) / Ammonium Ions (NH₄⁺) → (Nitrification) → Nitrite (NO₂⁻) → Nitrate (NO₃⁻) → (Denitrification) → Nitrogen (N₂)
Significance of the Nitrogen Cycle
- Supports plant growth: Provides essential nitrogen for proteins, DNA, and chlorophyll.
- Regulates atmospheric nitrogen: Prevents excessive buildup of nitrogen compounds in the environment.
- Maintains ecosystem balance: Ensures continuous recycling of nitrogen between abiotic and biotic components.
- Influences agriculture: Fertilization practices depend on understanding nitrogen dynamics.
- Affects global climate: Nitrous oxide (N₂O), a byproduct of denitrification, is a potent greenhouse gas.
Human Impact on the Nitrogen Cycle
- Excessive fertilizer use leads to nitrogen runoff, causing water pollution and algal blooms.
- Burning fossil fuels releases nitrogen oxides (NOx), contributing to air pollution and acid rain.
- Deforestation and soil degradation reduce nitrogen availability in ecosystems.
Methane Cycle (Gaseous Cycle):
Methane (CH₄) is a highly potent greenhouse gas, significantly more effective at trapping heat in the atmosphere than carbon dioxide (CO₂). However, unlike CO₂, which can persist in the atmosphere for centuries, methane has a relatively short atmospheric lifespan of about 12 years. Despite its shorter duration, methane plays a critical role in climate change due to its intense warming potential.
Methane also contributes to the formation of ground-level ozone, a harmful air pollutant that can cause respiratory issues in humans and damage vegetation. Although methane is naturally produced through the decomposition of organic matter, human activities have drastically increased its atmospheric concentration, leading to widespread environmental concerns.
Natural Sources of Methane Emissions
1. Wetlands: The Largest Natural Methane Source
Wetlands are the most significant natural source of methane, responsible for approximately 80% of global methane emissions from natural sources. These emissions are produced by methanogens, a group of microorganisms belonging to the archaea domain.
- Methanogens thrive in hypoxic (low-oxygen) environments, breaking down organic material and producing methane as a metabolic by-product.
- Wetlands such as swamps, bogs, marshes, and floodplains provide the ideal conditions for methanogens, contributing to the continuous release of methane into the atmosphere.
2. Termites: Small Insects with a Big Impact
Termites, despite their small size, are surprisingly significant methane producers.
- Inside their digestive systems, termites host microbes that facilitate anaerobic fermentation, a process that generates methane.
- Due to their detritivorous (decomposing organic matter) diet, termites play an essential role in the decomposition of plant material, indirectly influencing global methane emissions.
3. Oceans: A Complex Source of Methane
Methane emissions from oceans are still not fully understood, but researchers have identified two primary sources:
- Anaerobic digestion in marine organisms: Zooplankton and fish contribute to methane production through microbial fermentation within their digestive systems.
- Methane release from sediments and coastal drainage areas: Organic matter trapped in seafloor sediments undergoes anaerobic decomposition, generating methane that can eventually escape into the water column and atmosphere.
4. Methane Hydrates: A Dormant but Dangerous Reservoir
Methane hydrates, also called clathrates, are frozen methane deposits found in oceanic sediments and permafrost. These hydrates form under high-pressure, low-temperature conditions, where methane gas becomes trapped within a lattice of water molecules, creating ice-like crystalline structures.
- These deposits represent a massive reservoir of methane, but they remain stable only under specific pressure and temperature conditions.
- Climate change poses a serious threat—rising temperatures could destabilize methane hydrates, leading to sudden methane releases that could contribute to mass extinction events.
- Extracting methane hydrates is not feasible, as the change in pressure and temperature would cause the methane to escape before it could be harnessed.
Human-Caused Methane Emissions
Human activities are responsible for 50-65% of total global methane emissions, with three primary contributors:
Sector | Percentage of Global Methane Emissions |
Agriculture | 40% |
Fossil Fuels | 35% |
Waste | 20% |
The United Nations Environment Programme (UNEP) has urged nations to reduce methane emissions by 45% by 2030 to mitigate climate change. Specific recommendations vary by country. For instance, in India, UNEP emphasizes reducing methane emissions in the waste sector through improved sewage and landfill management.
1. Agriculture: The Largest Contributor
Agricultural activities account for 40% of human-induced methane emissions, mainly from livestock farming and rice cultivation.
A. Livestock and Enteric Fermentation
- Cattle, buffalo, sheep, and goats generate methane during digestion through microbial fermentation in their stomachs (rumen).
- This methane is expelled via exhalation and belching, making livestock farming one of the most significant methane sources.
- Mitigation strategies include dietary adjustments (e.g., feeding livestock seaweed-based supplements) and improved manure management.
B. Rice Cultivation: Waterlogged Fields and Methane
- Flooded rice paddies create oxygen-deficient conditions that promote methane-producing microbes.
- Methane is generated as organic material decomposes in submerged soil.
- Sustainable rice farming techniques, such as alternate wetting and drying (AWD), can significantly reduce methane emissions while maintaining crop yields.
2. Fossil Fuel Industry: A Major Polluter
The fossil fuel industry contributes 35% of global methane emissions, mainly through:
- Natural gas production and transport: Methane, the primary component of natural gas, leaks into the atmosphere during extraction, storage, and distribution.
- Coal mining: Methane trapped in coal seams (coalbed methane) is released during mining operations.
- Oil extraction and refining: Methane escapes during oil drilling, venting, and flaring.
Reducing methane emissions from fossil fuels involves detecting and sealing gas leaks, reducing flaring, and transitioning to cleaner energy sources.
3. Waste Management: Landfills and Sewage
Human waste contributes 20% of methane emissions, mainly through landfills and wastewater treatment.
A. Landfills: Decomposing Waste and Methane Emissions
- When organic waste decomposes without oxygen, methane is produced.
- Moisture and waste quantity influence the rate of methane generation.
- Mitigation strategies: Capturing landfill gas for energy production and reducing organic waste through composting.
B. Wastewater Treatment: Hidden Methane Emissions
- Anaerobic processing of organic matter in sewage treatment plants leads to methane emissions.
- Implementing anaerobic digesters can convert methane into biogas, which can be used for energy.
4. Biomass Burning: A Source of Incomplete Combustion
- Burning forests, grasslands, and agricultural residues produces methane.
- Wildfires and slash-and-burn agriculture are significant sources.
- Preventing deforestation and adopting controlled burning techniques can help reduce emissions.
Methane Sinks: How the Earth Absorbs Methane
1. Soil Methane Oxidation
- Certain methanotrophic bacteria in soil consume methane as an energy source.
- This natural process, known as methane oxidation, helps reduce atmospheric methane levels.
2. Hydroxyl Radical (OH) Reactions in the Atmosphere
- The hydroxyl radical (OH) acts as a “detergent” of the atmosphere, breaking down methane into carbon dioxide (CO₂) and water vapor.
- This reaction primarily occurs in the troposphere and stratosphere, helping to regulate methane levels.
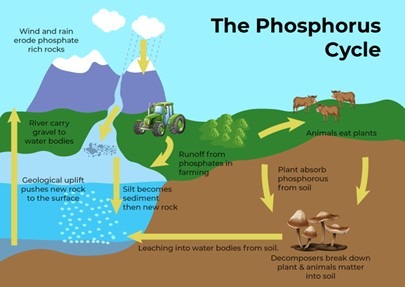
Phosphorous Cycle (Sedimentary Cycle):
Unlike carbon and nitrogen, phosphorus does not have a gaseous phase in its natural cycle. Instead, it moves through rocks, soil, water, and living organisms in what is known as the sedimentary cycle.
1. Role in Ecosystems
- Phosphorus is essential for DNA, RNA, ATP (energy molecules), and cell membranes.
- In aquatic ecosystems, excess phosphorus can promote harmful algal blooms, degrading water quality.
2. Sources and Entry into Ecosystems
- Unlike carbon and nitrogen, phosphorus originates from phosphate rocks.
- Phosphorus enters ecosystems through erosion, weathering, and human activities like mining.
3. Geological Storage and Ocean Accumulation
- Phosphorus accumulates as sediments on continental shelves, remaining trapped for millions of years.
- Tectonic uplift eventually exposes these deposits, reintroducing phosphorus into terrestrial ecosystems.
4. The Geochemical Cycle
- This cycle operates over millions of years, ensuring phosphorus is continuously recycled between land, water, and living organisms.
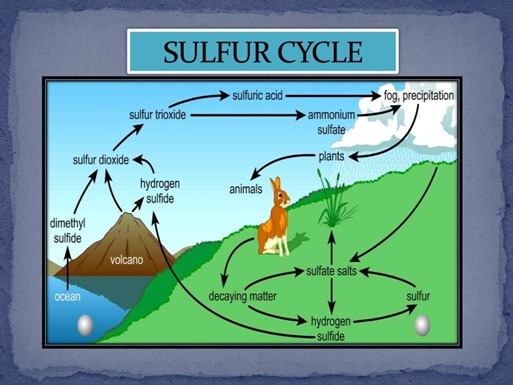
Sulphur Cycle:
Sulphur (S) is an essential element for life, playing a crucial role in various biological and geological processes. It is a key component of proteins, enzymes, and vitamins in living organisms and is involved in maintaining the Earth’s climate and atmospheric chemistry. The sulphur cycle is the continuous movement of sulphur between the biosphere, lithosphere, hydrosphere, and atmosphere, ensuring its availability for biological and chemical processes.
1. Sulphur in Soil and Sediments: Forms and Reservoirs
- Sulphur is found in soil and sediments in both organic and inorganic forms, primarily as:
- Sulphur-rich rocks (e.g., gypsum, pyrite, and anhydrite)
- Coal deposits
- Sulphates (SO₄²⁻) in soil and water
- Organic matter containing sulphur compounds
- These reservoirs act as long-term storage, slowly releasing sulphur into the environment through weathering, erosion, and decomposition.
2. The Gaseous Component of the Sulphur Cycle
- Although most sulphur is found in solid or dissolved forms, some gaseous compounds contribute to atmospheric processes. These include:
- Hydrogen sulphide (H₂S): A colourless, foul-smelling gas released during organic matter decomposition in anaerobic (low-oxygen) environments.
- Sulphur dioxide (SO₂): A key atmospheric sulphur compound produced naturally by volcanic eruptions, forest fires, and ocean emissions.
- Dimethyl sulphide (DMS, (CH₃)₂S): Emitted by marine phytoplankton, influencing cloud formation and climate regulation.
- These gases eventually return to Earth’s surface through precipitation or chemical transformations.
3. Natural and Human-Induced Sulphur Emissions
A. Natural Sources of Sulphur
- Several natural processes release sulphur into the environment, including:
- Volcanic Eruptions: Emit large amounts of SO₂ and H₂S into the atmosphere.
- Decomposition of Organic Matter: Anaerobic bacteria break down organic material, releasing H₂S into the environment.
- Oceans and Marine Ecosystems: The emission of dimethyl sulphide (DMS) contributes to the formation of aerosols and clouds.
- Weathering of Sulphur-Rich Rocks: Releases sulphates into soil and water bodies, making them available for biological use.
B. Human Activities and Sulphur Pollution
- Human activities have significantly altered the natural sulphur cycle, increasing atmospheric sulphur levels through:
- Burning of Fossil Fuels: Coal, oil, and natural gas combustion releases large amounts of SO₂, contributing to air pollution.
- Industrial Processes: Metal smelting (e.g., copper, zinc, and lead processing) produces significant sulphur emissions.
- Deforestation and Land Use Changes: Disturbance of soil sulphur reservoirs accelerates sulphur release.
- Excessive sulphur emissions from human activities have led to acid rain, environmental degradation, and respiratory health issues.
4. Sulphur Deposition: From Atmosphere to Land and Water
- Once in the atmosphere, sulphur undergoes chemical transformations and eventually returns to the Earth’s surface through wet and dry deposition.
- Wet Deposition: Sulphur dioxide (SO₂) reacts with water vapour to form sulphuric acid (H₂SO₄), which falls as acid rain.
- Dry Deposition: Sulphur compounds settle onto surfaces as fine particulate matter, affecting soil and vegetation.
- Environmental Consequences of Acid Rain:
- Soil Acidification: Lowers soil pH, reducing nutrient availability and harming plant life.
- Water Body Acidification: Leads to fish kills and loss of aquatic biodiversity.
- Damage to Buildings and Infrastructure: Acid rain corrodes stone structures, monuments, and metal surfaces.
5. Sulphur Assimilation and the Food Web
A. Plant Uptake and Incorporation
- Plants absorb sulphur primarily in the form of sulphates (SO₄²⁻) from soil and water. This sulphur is incorporated into:
- Amino acids (e.g., cysteine, methionine)
- Proteins, vitamins (e.g., biotin, thiamine), and coenzymes
- These sulphur-containing compounds are essential for plant growth and are transferred through the food chain as herbivores and carnivores consume plants and other organisms.
B. Decomposition and Sulphur Recycling
- When plants and animals die, their organic matter is broken down by decomposers (bacteria and fungi). This process releases sulphur back into the soil, water, and atmosphere, ensuring its continuous recycling.
6. Sulphur in Marine Ecosystems
- Sulphur plays a critical role in marine environments, particularly through:
- Dimethyl Sulphide (DMS) Production: Marine phytoplankton produce DMS, which helps regulate cloud formation and global climate.
- Sulphur Sediments: Ocean sediments store large amounts of sulphur in gypsum (CaSO₄) and pyrite (FeS₂).
- Deep-Sea Hydrothermal Vents: Rich in sulphur compounds, these ecosystems support unique chemosynthetic organisms that use H₂S as an energy source.
7. The Sulphur Cycle and Climate Regulation
- Sulphur compounds, particularly SO₂ and DMS, influence climate by affecting cloud formation and atmospheric reflectivity.
- DMS from Oceans: Helps form cloud condensation nuclei, increasing cloud cover and reducing solar radiation reaching Earth’s surface.
- SO₂ and Aerosols: Reflect sunlight, temporarily cooling the Earth’s surface (e.g., after volcanic eruptions).
- However, excessive human-induced SO₂ emissions disrupt natural climate processes, contributing to air pollution and acid rain formation.
8. The Future of Sulphur Management
- To mitigate the negative impacts of human-induced sulphur emissions, various strategies can be implemented:
A. Reducing Industrial Sulphur Emissions
- Switching to low-sulphur fuels to reduce SO₂
- Installing scrubbers in power plants to capture sulphur before it is released into the air.
- Encouraging cleaner energy alternatives such as wind, solar, and nuclear power.
B. Combating Acid Rain
- Regulating industrial emissions through international policies (e.g., the Clean Air Act).
- Using alkaline substances (e.g., lime) to neutralize acidified soils and water bodies.
- Reforestation and soil conservation to restore damaged ecosystems.
C. Sustainable Agriculture Practices
- Avoiding overuse of sulphur-rich fertilizers, which can contribute to soil and water pollution.
- Promoting crop rotation and soil conservation techniques to maintain natural sulphur balance.